Is Gasoline Burning A Chemical Change
Muz Play
Mar 19, 2025 · 5 min read
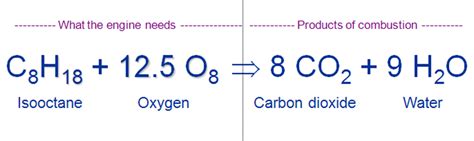
Table of Contents
Is Gasoline Burning a Chemical Change? A Deep Dive into Combustion
Burning gasoline, the lifeblood of countless vehicles worldwide, is far more than just a simple process of fuel disappearing. It’s a complex chemical reaction, a captivating example of combustion that underpins much of modern society. This article will delve deep into the chemical changes that occur when gasoline burns, exploring the underlying principles, the products formed, and the broader implications of this ubiquitous reaction.
Understanding Chemical Changes
Before we tackle the specifics of gasoline combustion, let's establish a clear understanding of what constitutes a chemical change. A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. This rearrangement is often accompanied by observable changes, such as a change in color, temperature, odor, or the formation of a precipitate (solid). Crucially, chemical changes are irreversible; you cannot easily revert the new substances back to their original forms. In contrast, a physical change alters the appearance of a substance without changing its chemical composition. For example, melting ice is a physical change; it's still H₂O, just in a different state.
Key Indicators of Chemical Change
Several key indicators can help identify a chemical change:
- Formation of a gas: The release of bubbles or fumes is a strong indication of a chemical reaction.
- Formation of a precipitate: The appearance of a solid from a solution signals a chemical reaction has occurred.
- Change in color: A significant alteration in the color of a substance often indicates a chemical change.
- Change in temperature: The release or absorption of heat (exothermic or endothermic reaction) is a common characteristic of chemical reactions.
- Change in odor: The production of a new smell points to the formation of new chemical compounds.
- Irreversibility: The inability to easily reverse the changes back to their original state strongly suggests a chemical reaction.
The Chemistry of Gasoline Combustion
Gasoline itself isn't a single compound; it's a complex mixture of hydrocarbons, primarily alkanes, with varying numbers of carbon atoms. These hydrocarbons range from relatively small molecules like butane (C₄H₁₀) to larger molecules like octane (C₈H₁₈). The precise composition of gasoline varies depending on factors such as the crude oil source and refining processes.
When gasoline burns, it undergoes a rapid oxidation reaction with oxygen (O₂ from the air). This is a classic example of combustion, a rapid chemical reaction that produces heat and light. The general equation for the complete combustion of a hydrocarbon (represented here by CₓHᵧ) is:
CₓHᵧ + (x + y/4)O₂ → xCO₂ + (y/2)H₂O + Heat + Light
This equation shows that hydrocarbons react with oxygen to produce carbon dioxide (CO₂), water (H₂O), heat, and light. The "x" and "y" represent the number of carbon and hydrogen atoms in the specific hydrocarbon molecule.
Complete vs. Incomplete Combustion
The equation above depicts complete combustion. This ideal scenario requires sufficient oxygen to fully oxidize all the hydrocarbons in the gasoline. However, in reality, complete combustion rarely occurs perfectly. If there's insufficient oxygen, incomplete combustion takes place, leading to the formation of other products, including:
- Carbon monoxide (CO): A highly toxic gas.
- Soot (carbon particles): Unburned carbon that contributes to air pollution.
- Unburned hydrocarbons: These contribute to smog formation.
Incomplete combustion is less efficient, releasing less energy and producing harmful pollutants. The ratio of fuel to oxygen is critical in determining whether combustion is complete or incomplete.
The Stages of Gasoline Combustion in an Engine
The combustion process in a gasoline engine is a highly controlled and complex sequence of events:
- Intake Stroke: Air and fuel are drawn into the cylinder.
- Compression Stroke: The mixture of air and fuel is compressed, increasing its temperature and pressure.
- Combustion (Power Stroke): A spark plug ignites the compressed fuel-air mixture, initiating rapid combustion. This is where the chemical changes described above take place. The expansion of gases drives the piston downwards, generating power.
- Exhaust Stroke: The exhaust gases (CO₂, H₂O, potentially CO, unburned hydrocarbons, and soot) are expelled from the cylinder.
The precise timing and control of these stages are crucial for efficient and clean combustion. Modern engines employ advanced technologies like fuel injection and catalytic converters to optimize combustion and minimize emissions.
Environmental Implications of Gasoline Combustion
The combustion of gasoline, while essential for transportation, has significant environmental consequences. The primary concerns stem from the release of:
- Greenhouse gases: The combustion of gasoline releases large amounts of carbon dioxide (CO₂), a potent greenhouse gas that contributes to global warming and climate change.
- Air pollutants: Incomplete combustion produces pollutants such as carbon monoxide (CO), nitrogen oxides (NOx), particulate matter (PM), and unburned hydrocarbons, which harm human health and the environment. These pollutants contribute to smog, acid rain, and respiratory problems.
Alternatives and Future Directions
The environmental impact of gasoline combustion has spurred research into alternative fuels and engine technologies. These include:
- Biofuels: Fuels derived from renewable biomass sources.
- Electric vehicles: Vehicles powered by electricity, eliminating direct combustion of gasoline.
- Hybrid vehicles: Vehicles that combine gasoline engines with electric motors.
- Hydrogen fuel cells: These generate electricity through a chemical reaction between hydrogen and oxygen, producing only water as a byproduct.
These alternatives offer the potential for cleaner and more sustainable transportation, gradually reducing the reliance on gasoline combustion and its associated environmental consequences.
Conclusion: Beyond a Simple Burn
The burning of gasoline is far more than a simple act of burning fuel. It's a complex chemical process, a fascinating example of combustion that involves numerous chemical reactions and produces a range of products. Understanding the chemistry of gasoline combustion is vital for developing cleaner and more efficient engines and for mitigating the environmental consequences of this ubiquitous energy source. The quest for cleaner and sustainable transportation continues, driven by the need to balance our reliance on gasoline with the urgent need to protect our planet. Further research and technological advancements are essential to achieving this critical balance for a sustainable future. The transition away from solely relying on gasoline combustion is not just desirable, but a necessary step towards a healthier environment for generations to come.
Latest Posts
Latest Posts
-
An Atom That Has Gained Or Lost Electrons
Mar 20, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Mar 20, 2025
-
How Many Covalent Bonds Does Hydrogen Have
Mar 20, 2025
-
Oxidation Of An Aldehyde Produces A
Mar 20, 2025
-
Evidence That Light Is A Particle
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Gasoline Burning A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
