Kinetics Of An Iodine Clock Reaction
Muz Play
Mar 25, 2025 · 6 min read
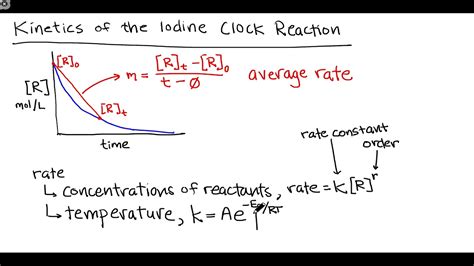
Table of Contents
The Kinetics of an Iodine Clock Reaction: A Deep Dive
The iodine clock reaction is a classic chemistry experiment demonstrating the principles of chemical kinetics. Its dramatic visual effect – a sudden color change from clear to dark blue – captivates students and provides a fascinating platform for exploring reaction rates, rate laws, and reaction mechanisms. This article will delve into the intricacies of this reaction, exploring its kinetics in detail, examining the factors influencing its rate, and outlining experimental techniques for studying it.
Understanding the Reaction
The iodine clock reaction typically involves the oxidation of iodide ions (I⁻) by hydrogen peroxide (H₂O₂) in an acidic solution. This reaction is relatively slow, but its progress can be monitored using a second, much faster reaction that involves the reduction of iodine (I₂) by thiosulfate ions (S₂O₃²⁻). The interplay between these two reactions creates the "clock" effect.
The overall reaction can be broken down into two key steps:
Step 1: The Slow Reaction
This is the rate-determining step of the reaction. Here, hydrogen peroxide oxidizes iodide ions to form iodine:
H₂O₂ + 2I⁻ + 2H⁺ → I₂ + 2H₂O
This reaction proceeds relatively slowly, and the rate depends on the concentrations of H₂O₂, I⁻, and H⁺ ions.
Step 2: The Fast Reaction
As iodine (I₂) is produced in the first step, it immediately reacts with thiosulfate ions (S₂O₃²⁻) in a rapid reaction:
I₂ + 2S₂O₃²⁻ → 2I⁻ + S₄O₆²⁻
This reaction consumes the iodine produced in the first step, keeping the solution colorless. The iodine concentration remains effectively zero until all the thiosulfate is consumed.
The "Clock" Mechanism
The "clock" aspect of the reaction arises because once all the thiosulfate is used up, the iodine produced in the first step is no longer consumed by the second step. This leads to a rapid increase in the concentration of iodine, which reacts with starch (often added as an indicator) to form a dark blue starch-iodine complex. The sudden appearance of this blue color marks the "clock" moment, indicating the completion of the thiosulfate reaction and the accumulation of a measurable amount of iodine.
Factors Affecting the Reaction Rate
Several factors influence the rate of the iodine clock reaction. Understanding these factors is crucial for a thorough kinetic analysis. Let's explore some of the most significant ones:
1. Concentration of Reactants
The rate of the reaction is directly proportional to the concentrations of the reactants in the rate-determining step (Step 1). Increasing the concentration of H₂O₂, I⁻, or H⁺ will increase the rate of the reaction, leading to a shorter time before the color change occurs. Experimental variations in these concentrations allow for the determination of the reaction order with respect to each reactant.
2. Temperature
Temperature significantly impacts reaction rates. Increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, thus accelerating the reaction. This effect can be quantified using the Arrhenius equation, allowing for the determination of the activation energy (Ea) of the reaction.
3. Presence of a Catalyst
The rate of the reaction can be significantly altered by the presence of a catalyst. Catalysts provide an alternative reaction pathway with a lower activation energy, thus increasing the reaction rate without being consumed in the process. Investigating the effects of various catalysts can provide insights into the reaction mechanism and the role of specific chemical species.
4. Ionic Strength
The ionic strength of the solution can also influence the reaction rate. Changes in ionic strength can affect the activity coefficients of the ions involved in the reaction, leading to changes in the effective concentrations and, consequently, the reaction rate.
Determining the Rate Law
The rate law for the iodine clock reaction can be determined experimentally by systematically varying the concentrations of the reactants and measuring the time taken for the color change to occur. The rate law generally takes the form:
Rate = k[H₂O₂]ˣ[I⁻]ʸ[H⁺]ᶻ
where:
kis the rate constantx,y, andzare the reaction orders with respect to H₂O₂, I⁻, and H⁺, respectively.
These reaction orders can be determined using the method of initial rates, where the initial concentrations of reactants are varied and the initial rates are measured. By comparing the initial rates at different concentrations, the reaction orders can be deduced.
Experimental Techniques
Studying the kinetics of the iodine clock reaction often involves precise measurements and careful experimental design. Here are some key techniques:
1. Spectrophotometry
Spectrophotometry is a powerful tool for monitoring the reaction progress in real-time. By measuring the absorbance of the solution at a specific wavelength (where the starch-iodine complex absorbs strongly), the concentration of iodine can be determined as a function of time. This allows for a more precise determination of the reaction rate compared to simply observing the color change.
2. Titration
After the color change, the remaining concentration of thiosulfate can be determined using titration with a standard iodine solution. This provides an alternative method for calculating the amount of iodine produced and helps in confirming the rate calculations obtained through other methods.
3. Temperature Control
Maintaining a constant temperature throughout the experiment is crucial for accurate kinetic studies. A thermostatically controlled water bath is often used to ensure that the temperature remains stable during the reaction.
4. Data Analysis
The experimental data obtained (time, concentration, absorbance) needs to be carefully analyzed to determine the rate law, rate constant, and activation energy. Graphical methods, such as plotting concentration versus time or ln(rate) versus 1/T (for activation energy determination), are commonly employed for data interpretation.
Applications and Significance
The iodine clock reaction is not just a fascinating classroom demonstration; it holds significant value in various fields:
- Education: It provides a hands-on, engaging way to teach fundamental concepts in chemical kinetics, including reaction rates, rate laws, reaction orders, and activation energy.
- Research: Modified versions of the iodine clock reaction have been used in research to study the kinetics of complex reaction systems and to develop new catalytic materials.
- Analytical Chemistry: The reaction's sensitivity to changes in reactant concentrations allows it to be adapted for various analytical applications.
Conclusion
The iodine clock reaction serves as a powerful tool for illustrating the fundamental principles of chemical kinetics. Its visually striking nature and the ability to manipulate several variables to affect reaction rates make it a valuable experiment for both educational and research purposes. By understanding the factors influencing its kinetics and employing appropriate experimental techniques, one can gain valuable insights into reaction mechanisms and develop a deeper appreciation for the dynamic nature of chemical processes. Further exploration of this reaction can involve investigating the effects of different catalysts, solvents, and reaction conditions, pushing the boundaries of our understanding of chemical reactivity. The seemingly simple color change hides a wealth of intricate chemical processes, making it a truly captivating and insightful experiment.
Latest Posts
Latest Posts
-
What Is The Relationship Between Avogadros Number And The Mole
Mar 28, 2025
-
What Are The Building Blocks For Fats
Mar 28, 2025
-
The Si Unit Of Energy Is The
Mar 28, 2025
-
Water Molecules Move Across Cells By
Mar 28, 2025
-
Is Ammonium Hydroxide A Strong Base
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Kinetics Of An Iodine Clock Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
