Similarities Between Ionic And Covalent Compounds
Muz Play
Mar 28, 2025 · 6 min read
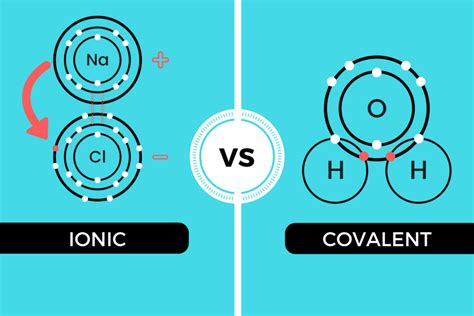
Table of Contents
Unveiling the Similarities Between Ionic and Covalent Compounds: A Deep Dive
Ionic and covalent compounds, the fundamental building blocks of chemistry, are often presented as stark opposites. While their contrasting mechanisms of bond formation—electrostatic attraction versus electron sharing—are crucial, a closer examination reveals surprising similarities that bridge the apparent divide. This article will delve into the unexpected commonalities between these seemingly disparate compound types, exploring their structural features, physical properties, and even their roles in biological systems.
Beyond the Differences: Shared Ground in Chemical Bonding
The core difference lies in how atoms achieve stability. Ionic compounds achieve stability by transferring electrons, creating positively charged cations and negatively charged anions that are held together by strong electrostatic forces. Covalent compounds, on the other hand, achieve stability by sharing electrons between atoms, forming a more equitable distribution of charge. However, the reality is more nuanced. The distinction isn’t always clear-cut; many compounds exhibit characteristics of both ionic and covalent bonding, blurring the lines between categories.
Polarity: A Common Thread
One striking similarity is the concept of polarity. While often associated with covalent bonds, ionic bonds can also exhibit polarity. In perfectly ionic compounds, the electron transfer is complete, resulting in a purely ionic bond. However, in reality, complete electron transfer is rare. The degree of electron transfer depends on the electronegativity difference between the atoms involved. A larger electronegativity difference leads to a more ionic character, while a smaller difference results in a more covalent character. This leads to the concept of polar covalent bonds, where electrons are unequally shared, creating a dipole moment. Similarly, even in seemingly purely ionic compounds, the distribution of electron density around the ions isn't perfectly uniform, leading to some degree of polarization.
Solubility and Conductivity: Overlapping Behaviors
Solubility and conductivity are often used to distinguish ionic and covalent compounds. Ionic compounds are generally soluble in polar solvents like water, due to the strong interaction between the ions and the polar solvent molecules. Covalent compounds, particularly nonpolar ones, are typically soluble in nonpolar solvents. However, many exceptions exist. Some covalent compounds, such as sugars and certain alcohols, are highly soluble in water due to the presence of polar functional groups that can interact with water molecules through hydrogen bonding.
Furthermore, while ionic compounds typically conduct electricity when molten or dissolved in water (due to the presence of mobile ions), some covalent compounds can also conduct electricity under specific conditions. For example, certain molten covalent compounds, such as aluminum chloride (AlCl₃), exhibit conductivity due to the formation of ions. The conductivity depends on the degree of ionization or dissociation that occurs in the molten or dissolved state. This further underlines the gray areas between these seemingly distinct categories.
Structural Similarities and Exceptions: Beyond the Simple Model
The traditional models of ionic and covalent compounds—simple lattices for ionic compounds and discrete molecules for covalent compounds—are oversimplifications. The reality often involves more complex structures.
Network Covalent Compounds: Challenging the Discrete Molecule Notion
Network covalent compounds, such as diamond and quartz (SiO₂), challenge the notion that all covalent compounds exist as discrete molecules. In these compounds, atoms are bonded covalently in a continuous three-dimensional network, resulting in extremely high melting points and hardness. This network structure, while driven by covalent bonding, shares some similarities with the extended three-dimensional lattices seen in ionic compounds. Both types of compounds exhibit a high degree of structural order and are generally solid at room temperature.
Polymeric Structures: Bridging the Gap
Polymeric structures also bridge the gap between ionic and covalent compounds. Polymers can be formed through covalent bonds between repeating monomer units, creating long chains or networks. However, ionic interactions can also play a significant role in the overall structure and properties of polymers. For example, the interactions between charged groups along the polymer chains can influence their solubility, flexibility, and other properties. This complex interplay of covalent and ionic interactions highlights the interconnectedness of bonding mechanisms.
Physical Properties: A Spectrum of Behavior
While differences in melting and boiling points are often used to distinguish ionic and covalent compounds (ionic compounds generally having higher melting points due to stronger electrostatic interactions), this isn't a hard and fast rule. The physical properties of both types depend significantly on factors like the size of the atoms/ions, the strength of the bonds, and the presence of intermolecular forces.
Intermolecular Forces: A Crucial Factor
Intermolecular forces, like hydrogen bonding, dipole-dipole interactions, and London dispersion forces, influence the physical properties of both ionic and covalent compounds. These forces, while weaker than ionic or covalent bonds, play a crucial role in determining the melting points, boiling points, and solubility of compounds. The strength of intermolecular forces depends on factors like molecular shape and polarity, affecting both ionic and covalent compounds. For instance, the high boiling point of water, a covalent compound, is due to strong hydrogen bonding between water molecules.
Melting and Boiling Points: A Continuum of Behavior
Melting and boiling points aren't solely determined by the primary bonding type. The strength of intermolecular forces plays a crucial role. A large covalent molecule with strong intermolecular forces may have a higher melting point than a smaller ionic compound with weaker interactions. This highlights the importance of considering the interplay of different forces when predicting physical properties.
Biological Significance: An Intertwined Role
Both ionic and covalent compounds play vital roles in biological systems. Covalent bonds form the backbone of biological macromolecules like proteins, nucleic acids, and carbohydrates, determining their structure and function. Ionic interactions are crucial for maintaining the three-dimensional structure of proteins and for the interaction between biological molecules, such as enzyme-substrate interactions. The interplay between these bonding mechanisms is essential for the complex functions of living organisms.
Electrolyte Balance: The Importance of Ions
Ions, arising from the dissociation of ionic compounds, play a critical role in maintaining electrolyte balance in biological systems. This balance is essential for nerve impulse transmission, muscle contraction, and many other physiological processes. The precise concentrations of ions like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) are carefully regulated in living organisms, highlighting the vital role of ionic compounds in biological function.
Conclusion: A Unified Perspective
While traditionally presented as distinct entities, ionic and covalent compounds share surprising similarities. The degree of electron transfer in bond formation exists on a spectrum, blurring the line between purely ionic and purely covalent bonds. The influence of intermolecular forces, the complexity of structural arrangements, and the overlapping roles in biological systems all point to a more unified perspective. Understanding these commonalities provides a deeper understanding of chemical bonding and the properties of matter, moving beyond simplistic categorization to appreciate the interconnectedness of chemical phenomena. The seemingly contrasting nature of ionic and covalent bonding is better understood as a continuum rather than a rigid dichotomy, leading to a more complete and nuanced picture of the chemical world.
Latest Posts
Latest Posts
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
-
Why Does Km Increase In Competitive Inhibition
Mar 31, 2025
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
-
When Do You Consider Log Diterminants Similar
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Similarities Between Ionic And Covalent Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
