To Decrease The Concentration Of A Solujtion Add More Liquid
Muz Play
Mar 22, 2025 · 6 min read
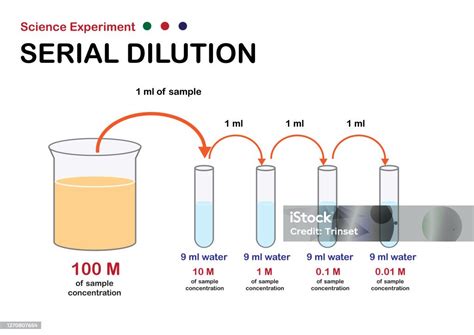
Table of Contents
Diluting Solutions: A Comprehensive Guide to Decreasing Concentration by Adding More Liquid
The concentration of a solution, whether it's a simple saline solution or a complex chemical mixture, is a crucial factor in many scientific and industrial applications. Understanding how to dilute a solution, the process of decreasing its concentration by adding more solvent, is therefore essential. This comprehensive guide will delve into the science behind dilution, explore various dilution techniques, discuss safety precautions, and provide practical examples to solidify your understanding.
Understanding Concentration and Dilution
Before we dive into the methods of dilution, let's establish a firm understanding of the core concepts. Concentration refers to the amount of solute (the substance being dissolved) present in a given amount of solvent (the substance doing the dissolving). This is often expressed as a ratio, such as grams per liter (g/L), moles per liter (mol/L) – also known as molarity (M), or percent by weight (% w/w).
Dilution is the process of decreasing the concentration of a solution by adding more solvent. Importantly, this process does not change the amount of solute present; it simply increases the total volume of the solution, thereby lowering the concentration of the solute within that volume. This is governed by the fundamental principle of conservation of mass: the amount of solute remains constant throughout the dilution process.
Different Ways to Express Concentration
Several methods exist for expressing the concentration of a solution. Choosing the appropriate method depends on the specific application and the nature of the solute and solvent. Here are a few common methods:
-
Molarity (M): Moles of solute per liter of solution. This is a very common unit in chemistry and is particularly useful when dealing with stoichiometric calculations.
-
Molality (m): Moles of solute per kilogram of solvent. This is less common than molarity but is advantageous because it's temperature-independent, unlike molarity.
-
Normality (N): Equivalents of solute per liter of solution. This is useful in acid-base titrations and other reactions involving equivalents.
-
Percent by Weight (% w/w): The mass of solute divided by the total mass of the solution, multiplied by 100.
-
Percent by Volume (% v/v): The volume of solute divided by the total volume of the solution, multiplied by 100. This is commonly used for solutions where both solute and solvent are liquids.
-
Parts per Million (ppm) and Parts per Billion (ppb): These units are used for extremely dilute solutions, representing the number of solute particles per million or billion particles of the solution.
Calculating Dilution: The Dilution Formula
The core principle behind dilution calculations is the conservation of the amount of solute. The formula to calculate the final concentration or volume after dilution is:
M1V1 = M2V2
Where:
- M1 is the initial concentration of the solution.
- V1 is the initial volume of the solution.
- M2 is the final concentration of the solution after dilution.
- V2 is the final volume of the solution after dilution.
This formula is incredibly versatile. You can use it to determine any of the four variables if you know the other three. For example, you can calculate the final concentration after adding a specific volume of solvent, or determine the volume of solvent needed to achieve a desired concentration.
Techniques for Diluting Solutions
The precise method for diluting a solution depends on the desired accuracy and the properties of the solution. Here are some common techniques:
1. Using a Volumetric Flask: A Precise Method
This method is ideal for preparing solutions with high accuracy. It involves transferring a precise volume of the stock solution (the concentrated solution) into a volumetric flask using a pipette. Then, the solvent is added carefully until the meniscus reaches the calibration mark on the flask's neck. The flask is then stoppered and inverted several times to ensure thorough mixing. This ensures a precisely known final volume and concentration.
2. Serial Dilution: Creating a Range of Concentrations
Serial dilutions are used to create a series of solutions with progressively decreasing concentrations. A small volume of the stock solution is transferred to a larger volume of solvent, creating a diluted solution. Then, a portion of this diluted solution is further diluted, and so on. This technique is very useful in applications such as creating calibration curves in analytical chemistry.
3. Direct Dilution: Simple and Quick
This involves directly adding solvent to a specific volume of stock solution. This is a less precise method than using a volumetric flask, but it's faster and suitable for applications where high precision isn't crucial. It's important to ensure thorough mixing to achieve a uniform concentration throughout the solution.
Safety Precautions When Diluting Solutions
Diluting solutions, while seemingly straightforward, can present safety hazards if proper precautions aren't taken. Remember:
-
Always wear appropriate personal protective equipment (PPE): This includes safety goggles, lab coats, and gloves. Some solutions are corrosive, toxic, or irritating, and direct contact should be avoided.
-
Add acid to water, never water to acid: This is a critical safety rule, especially when dealing with strong acids. Adding water to acid can cause a violent exothermic reaction, potentially leading to splashing and burns.
-
Work in a well-ventilated area: Some solutions release fumes that can be harmful if inhaled. A fume hood is recommended for volatile or toxic solutions.
-
Dispose of solutions properly: Follow all local regulations and guidelines for disposing of chemical waste. Never pour solutions down the drain unless specifically permitted.
-
Be aware of potential heat generation: Some dilutions can be exothermic, meaning they release heat. Be cautious when handling such solutions and allow them to cool before further manipulation.
Practical Examples of Dilution Calculations
Let's work through a few examples to illustrate the application of the dilution formula:
Example 1: You have 100 mL of a 2.0 M NaCl solution. You want to dilute it to a final concentration of 0.5 M. What will the final volume be?
Using the formula M1V1 = M2V2:
(2.0 M)(100 mL) = (0.5 M)(V2) V2 = (2.0 M * 100 mL) / 0.5 M = 400 mL
Therefore, the final volume will be 400 mL. You'll need to add 300 mL of solvent to the original 100 mL solution.
Example 2: You need 500 mL of a 0.1 M HCl solution. You have a stock solution of 1.0 M HCl. How much of the stock solution do you need?
Using the formula M1V1 = M2V2:
(1.0 M)(V1) = (0.1 M)(500 mL) V1 = (0.1 M * 500 mL) / 1.0 M = 50 mL
You need 50 mL of the 1.0 M HCl stock solution. You would add 450 mL of solvent to achieve the desired 500 mL of 0.1 M HCl.
Advanced Dilution Concepts: Dilution Factor and Serial Dilutions
Understanding dilution factors and mastering serial dilutions are crucial for advanced applications.
Dilution Factor: The dilution factor is the ratio of the final volume to the initial volume (V2/V1). It represents the extent of dilution. For instance, a dilution factor of 10 means the solution has been diluted tenfold.
Serial Dilutions: These are a series of dilutions where a portion of a diluted solution is further diluted. This allows for the creation of solutions with very low concentrations. It's crucial to accurately measure and mix each step to minimize errors.
Conclusion
Diluting solutions is a fundamental technique in many scientific and industrial contexts. Understanding the underlying principles, mastering the calculation methods, and adhering to safety protocols are essential for successful and safe operation. The techniques outlined in this guide, ranging from simple direct dilutions to precise volumetric flask methods and intricate serial dilutions, provide a comprehensive foundation for accurately manipulating solution concentrations. Remember that precision and safety are paramount when handling any chemical solution. Always double-check your calculations and prioritize safe laboratory practices.
Latest Posts
Latest Posts
-
How Does Sexual Reproduction Lead To Genetic Variation
Mar 23, 2025
-
Why Are Amino Acids Called Amino Acids
Mar 23, 2025
-
How Do You Find The Length Of A Vector
Mar 23, 2025
-
Lewis Structure Of Carbon Monoxide With Formal Charges
Mar 23, 2025
-
What Is A Kirby Bauer Test
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about To Decrease The Concentration Of A Solujtion Add More Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
