Two Oxygen Atoms Combine To Form A Molecule By Sharing
Muz Play
Mar 21, 2025 · 5 min read
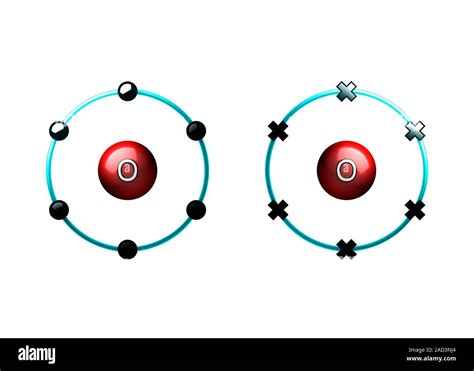
Table of Contents
Two Oxygen Atoms Combine to Form a Molecule by Sharing: A Deep Dive into Covalent Bonding
Oxygen, the life-giving element, exists predominantly as a diatomic molecule – O₂. This seemingly simple fact belies a fascinating process: the formation of a stable molecule through the sharing of electrons between two oxygen atoms. Understanding this process, known as covalent bonding, is fundamental to grasping the nature of chemical interactions and the properties of matter. This article will delve into the details of how two oxygen atoms combine to form an oxygen molecule, exploring the underlying principles of covalent bonding and its implications.
The Lone Oxygen Atom: An Unstable Entity
Before we dive into the bonding process, let's consider an individual oxygen atom. Oxygen, with atomic number 8, possesses eight electrons. Its electron configuration is 1s²2s²2p⁴. This means it has two electrons in the first energy level (1s orbital), two in the second energy level's s orbital (2s), and four in the second energy level's p orbitals (2p). Crucially, the outermost shell, the valence shell (2p), is incomplete. It needs two more electrons to achieve a stable octet – a full outermost shell, mirroring the electron configuration of the noble gas neon. This drive for stability is the key motivator behind oxygen's tendency to form covalent bonds.
The Importance of the Octet Rule
The octet rule, while not universally applicable, serves as a helpful guideline for predicting the bonding behavior of many atoms, including oxygen. Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their valence shell. This stable configuration provides lower energy, making the atom less reactive. For oxygen, achieving this stable octet is paramount.
The Dance of Electrons: Covalent Bond Formation
An oxygen atom cannot easily gain two electrons to achieve a stable octet because acquiring a negative charge requires significant energy. Losing electrons is similarly unfavorable. The solution lies in covalent bonding: the sharing of electrons between atoms.
When two oxygen atoms approach each other, their valence electrons interact. Specifically, each oxygen atom contributes two unpaired electrons from its 2p orbitals to the bond formation. These two pairs of electrons are then shared between the two oxygen atoms. This sharing creates a region of high electron density between the nuclei of the two oxygen atoms, forming a strong attractive force known as a covalent bond.
The Double Bond: Sharing Twice Over
The sharing isn't limited to one pair of electrons. Each oxygen atom shares two pairs of electrons with its partner, resulting in a double covalent bond. This double bond is significantly stronger than a single covalent bond, contributing to the relatively high stability and relatively high bond dissociation energy of the oxygen molecule. The double bond is represented visually with two lines connecting the oxygen atoms: O=O.
Delving Deeper: Molecular Orbital Theory
While the simple concept of electron sharing provides a good starting point, a more accurate and comprehensive understanding requires delving into molecular orbital (MO) theory. MO theory describes the formation of molecular orbitals by the combination of atomic orbitals.
In the case of oxygen, the 2s and 2p atomic orbitals of each oxygen atom combine to form molecular orbitals that encompass both atoms. These molecular orbitals are categorized as either bonding orbitals (lower energy, stabilizing the molecule) or antibonding orbitals (higher energy, destabilizing the molecule).
The electrons are then filled into these molecular orbitals according to the aufbau principle (filling lower energy orbitals first) and Hund's rule (maximizing electron spin before pairing). In the oxygen molecule, the bonding orbitals are filled, creating a stable diatomic molecule. However, the presence of electrons in antibonding orbitals slightly weakens the bond compared to what would be predicted by simple valence bond theory.
Paramagnetism: A Unique Property
An interesting consequence of the MO diagram of oxygen is its paramagnetism. Paramagnetism is a property exhibited by substances that are weakly attracted to a magnetic field. This arises from the presence of unpaired electrons in the antibonding orbitals. While the majority of electrons are paired, two electrons remain unpaired in the oxygen molecule's molecular orbital configuration, leading to this observable magnetic property.
Beyond the Basics: Implications of Covalent Bonding in Oxygen
The covalent bonding in oxygen has profound implications for its chemical and physical properties:
-
Gas at Room Temperature: The relatively weak intermolecular forces between O₂ molecules (London Dispersion Forces) mean oxygen exists as a gas at room temperature. This is crucial for its role in respiration.
-
Reactivity: While O₂ is a relatively stable molecule, it is still a highly reactive species, readily participating in oxidation reactions, which is essential for various biological processes and combustion. The double bond, while strong, is also susceptible to breaking, allowing oxygen to form new bonds with other atoms.
-
Biological Significance: The unique properties of oxygen, largely determined by its covalent bonding, are essential for life on Earth. Oxygen plays a critical role in cellular respiration, the process that provides energy for living organisms. The high reactivity of oxygen facilitates this energy-generating process.
-
Industrial Applications: Oxygen's reactivity is harnessed in numerous industrial processes, including combustion, welding, and the production of various chemicals.
Conclusion: A Simple Bond, a Complex Story
The seemingly simple act of two oxygen atoms sharing electrons to form a molecule reveals a wealth of complex chemical principles. From the fundamental concepts of the octet rule and covalent bonding to the more sophisticated molecular orbital theory, understanding the formation of the oxygen molecule illuminates the fundamental nature of chemical interactions. The double bond, the slight paramagnetism, and the resulting reactivity all contribute to oxygen's vital role in the world around us. This detailed look underscores the importance of learning basic chemistry, for it's through the understanding of these seemingly small interactions that we can appreciate the complexity and beauty of the natural world. The creation of an oxygen molecule, a process that occurs countless times every second, is a testament to the elegance and power of covalent bonding.
Latest Posts
Latest Posts
-
What Is The Activity Series In Chemistry
Mar 28, 2025
-
Solubility Of A Gas In A Liquid
Mar 28, 2025
-
Lewis Dot Structure Practice Worksheet Answers
Mar 28, 2025
-
What Is The Relationship Between Force And Mass
Mar 28, 2025
-
What Is The Mass Number Of An Atom Equal To
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Two Oxygen Atoms Combine To Form A Molecule By Sharing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
