Type Of Bond Of Sodium Chloride
Muz Play
Mar 28, 2025 · 6 min read
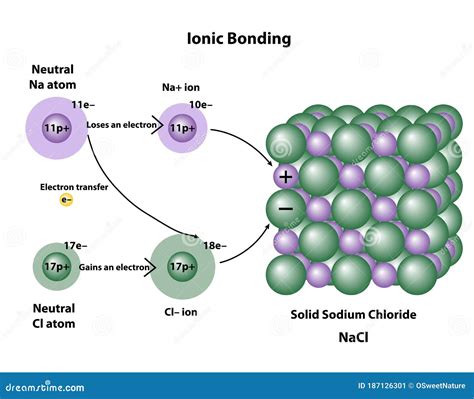
Table of Contents
The Ionic Bond in Sodium Chloride: A Deep Dive into Structure and Properties
Sodium chloride (NaCl), commonly known as table salt, is a ubiquitous compound with a simple yet fascinating chemical structure. Its properties, from its crystalline structure to its solubility in water, are all directly related to the type of bond holding its sodium (Na) and chlorine (Cl) atoms together: the ionic bond. This article delves deep into the nature of this ionic bond in NaCl, exploring its formation, characteristics, and implications on the macroscopic properties of the compound.
Understanding Ionic Bonds: A Foundation
Before exploring the specifics of the NaCl bond, it's crucial to understand the fundamental principles of ionic bonding. Ionic bonds form through the electrostatic attraction between oppositely charged ions. This means one atom loses electrons to become a positively charged ion (cation), and another atom gains those electrons to become a negatively charged ion (anion). The strong Coulombic forces between these ions hold the compound together.
Electronegativity: The Driving Force
The formation of ionic bonds is heavily influenced by the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Large differences in electronegativity between two atoms are crucial for ionic bond formation. In the case of NaCl, chlorine has a much higher electronegativity than sodium.
The Electron Transfer Process
The process begins with sodium, an alkali metal, possessing one electron in its outermost shell (valence shell). Chlorine, a halogen, needs one electron to complete its outermost shell. Sodium readily loses its valence electron to achieve a stable, filled electron shell (octet rule), becoming a positively charged sodium ion (Na⁺). Chlorine readily accepts this electron, gaining a stable octet and becoming a negatively charged chloride ion (Cl⁻).
The Ionic Bond in Sodium Chloride: A Detailed Look
The ionic bond in NaCl is the result of the electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion. This attraction is exceptionally strong, leading to the formation of a highly stable crystalline structure.
Lattice Energy: A Measure of Strength
The strength of the ionic bond in NaCl is quantified by its lattice energy. Lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. The high lattice energy of NaCl reflects the strong electrostatic forces holding the ions together. This high energy explains why NaCl has a high melting and boiling point, requiring significant energy input to overcome the attractive forces between the ions.
Crystal Structure: A Regular Arrangement
The arrangement of Na⁺ and Cl⁻ ions in NaCl is not random. Instead, they form a highly ordered cubic close-packed crystal lattice. This structure maximizes electrostatic attraction and minimizes electrostatic repulsion, leading to a highly stable and efficient arrangement. Each Na⁺ ion is surrounded by six Cl⁻ ions, and each Cl⁻ ion is surrounded by six Na⁺ ions. This specific arrangement gives rise to the characteristic cubic crystalline structure observed in NaCl crystals.
Properties Arising from Ionic Bonding in NaCl
The ionic bonding in NaCl directly influences a variety of its physical and chemical properties:
High Melting and Boiling Points:
The strong electrostatic forces between the ions require a significant amount of energy to overcome. This results in high melting (801°C) and boiling (1413°C) points for NaCl.
Brittleness:
NaCl crystals are brittle. When subjected to stress, the crystal lattice can shift, bringing ions of like charge into close proximity. This leads to strong electrostatic repulsion, causing the crystal to fracture.
Solubility in Water:
NaCl is highly soluble in water. Water molecules are polar, possessing a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. These polar water molecules effectively surround and solvate the Na⁺ and Cl⁻ ions, weakening the electrostatic attraction between them and allowing the salt to dissolve.
Electrical Conductivity:
Solid NaCl is a poor conductor of electricity because the ions are held rigidly in the crystal lattice and cannot move freely. However, when molten or dissolved in water, NaCl becomes a good conductor because the ions are free to move and carry an electric current.
Hardness:
Although brittle, NaCl crystals exhibit a certain degree of hardness due to the strong electrostatic forces holding the ions in place. This hardness is less pronounced than in covalent network solids like diamond, but still noticeable.
Comparing Ionic Bonds with Other Bond Types
Understanding the ionic bond in NaCl requires comparing it with other types of chemical bonds. These include:
Covalent Bonds:
Covalent bonds involve the sharing of electrons between atoms, unlike the electron transfer in ionic bonds. Covalent bonds typically occur between atoms with similar electronegativities. Examples include the bonds in molecules like methane (CH₄) and water (H₂O).
Metallic Bonds:
Metallic bonds involve the delocalized sharing of electrons among a lattice of metal atoms. This results in high electrical and thermal conductivity, malleability, and ductility, properties not seen in ionic compounds like NaCl.
The differences in electronegativity, electron sharing vs. transfer, and resulting properties clearly distinguish ionic bonds from covalent and metallic bonds. The ionic bond in NaCl is a prime example of a strong electrostatic interaction leading to a unique set of macroscopic properties.
Beyond the Basics: Advanced Concepts
The ionic model of NaCl, while accurate in many respects, is a simplification. Advanced concepts provide a more nuanced understanding:
Polarizability:
While primarily ionic, a small degree of covalent character exists in the NaCl bond due to the polarizability of the ions. Polarizability refers to the ease with which the electron cloud of an ion can be distorted. Although small, this effect influences properties like the solubility of NaCl.
Defects in the Crystal Lattice:
Real NaCl crystals aren't perfect. Defects, such as vacancies (missing ions) and interstitial ions (ions in between lattice sites), exist and can influence the physical properties, particularly electrical conductivity.
Applications:
The properties stemming from the ionic bond in NaCl make it vital in various applications:
- Food Preservation: NaCl inhibits microbial growth, making it a crucial preservative.
- De-icing: NaCl lowers the freezing point of water, used to melt ice on roads.
- Medicine: NaCl solutions are used in intravenous fluids and other medical applications.
- Industrial Processes: NaCl is a crucial raw material in many chemical industries, for example, in the production of chlorine and sodium hydroxide.
Conclusion
The ionic bond in sodium chloride is a foundational concept in chemistry, explaining the compound's unique properties and widespread applications. From its high melting point and brittle nature to its solubility in water and electrical conductivity in solution, all are direct consequences of the strong electrostatic attraction between the Na⁺ and Cl⁻ ions. A deep understanding of this bond type provides insight into the behavior of many other ionic compounds, highlighting the fundamental role of electronegativity and electrostatic forces in shaping the properties of matter. Further exploration of the advanced concepts surrounding crystal defects and polarizability offers an even more complete picture of this simple yet profoundly significant compound.
Latest Posts
Latest Posts
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
-
Why Does Km Increase In Competitive Inhibition
Mar 31, 2025
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
-
When Do You Consider Log Diterminants Similar
Mar 31, 2025
-
Find The Rectangular Equation And Eliminate The Parameters
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Type Of Bond Of Sodium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
