What Is The Electron Configuration Of Lithium
Muz Play
Mar 28, 2025 · 6 min read
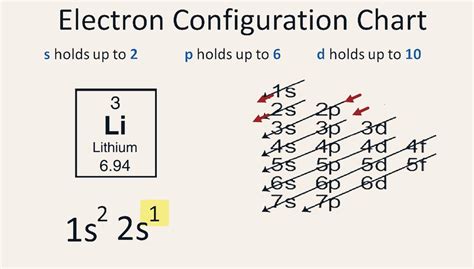
Table of Contents
What is the Electron Configuration of Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, holds a significant place in chemistry and beyond. Understanding its electron configuration is key to grasping its unique properties and reactivity. This article will explore the electron configuration of lithium in detail, explaining the underlying principles, its implications for chemical behavior, and delving into related concepts like orbital diagrams and quantum numbers.
Understanding Electron Configuration
Before diving into lithium's specific configuration, let's establish a foundational understanding of what electron configuration represents. Electron configuration describes the arrangement of electrons in the different energy levels and sublevels within an atom. It's essentially an atom's "address book" for its electrons, indicating where each electron resides. This arrangement is dictated by the principles of quantum mechanics and determines an atom's chemical properties.
Key Principles Governing Electron Configuration:
- Aufbau Principle: Electrons fill the lowest energy levels first. Think of it like filling a building from the ground floor up – you wouldn't start on the tenth floor!
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins. This is like having a maximum of two people per room, and they need to be distinguishable (opposite spins).
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is like each person getting their own room before sharing.
These principles work together to determine the most stable and lowest-energy electron configuration for any atom.
Determining Lithium's Electron Configuration
Lithium (Li) has an atomic number of 3, meaning it possesses three protons in its nucleus and, in a neutral atom, three electrons orbiting the nucleus. Using the Aufbau principle, we can systematically fill the energy levels:
- First energy level (n=1): This level contains only one subshell, the 1s subshell, which can hold a maximum of two electrons.
- Second energy level (n=2): This level contains the 2s and 2p subshells. The 2s subshell can hold two electrons, while the 2p subshell can hold six.
Following the Aufbau principle, we fill the lowest energy levels first. The two electrons fill the 1s orbital, and the remaining electron occupies the 2s orbital. Therefore, the electron configuration of lithium is 1s²2s¹.
Visualizing Electron Configuration: Orbital Diagrams
While the electron configuration provides a concise representation, orbital diagrams offer a more visual approach. They illustrate the individual orbitals and the electron occupancy within each. For lithium, the orbital diagram would look like this:
1s: ↑↓
2s: ↑
The arrows represent electrons, and their direction indicates the spin (up or down). The filled 1s orbital shows two electrons with opposite spins, while the partially filled 2s orbital has one electron.
Quantum Numbers and Lithium's Electrons
Each electron in an atom can be uniquely identified by a set of four quantum numbers:
- Principal quantum number (n): This describes the energy level of the electron (n=1, 2, 3...). For lithium's electrons, n=1 for the two 1s electrons and n=2 for the 2s electron.
- Azimuthal quantum number (l): This describes the subshell (or shape) of the orbital (l=0 for s, l=1 for p, l=2 for d...). For lithium, l=0 for both the 1s and 2s electrons.
- Magnetic quantum number (ml): This describes the orientation of the orbital in space (ml = -l, ..., 0, ..., +l). For s orbitals (l=0), ml=0.
- Spin quantum number (ms): This describes the intrinsic angular momentum of the electron (ms = +1/2 or -1/2). In lithium's 1s orbital, one electron has ms = +1/2 and the other has ms = -1/2. The 2s electron has either ms = +1/2 or -1/2.
These quantum numbers provide a complete description of each electron's state within the lithium atom.
Implications of Lithium's Electron Configuration for its Chemical Behavior
Lithium's electron configuration, 1s²2s¹, directly influences its chemical properties. The single electron in the 2s orbital is relatively loosely held and easily lost. This explains lithium's high reactivity and its tendency to form a +1 cation (Li⁺) by losing this outer electron. This cationic state is exceptionally stable due to the attainment of a noble gas configuration, resembling helium (1s²).
This single valence electron is responsible for lithium's characteristic reactions:
- Reaction with water: Lithium reacts vigorously with water, producing hydrogen gas and lithium hydroxide. The single electron is readily transferred to water molecules.
- Formation of ionic compounds: Lithium readily forms ionic compounds with nonmetals by donating its valence electron to achieve a stable octet. Examples include lithium chloride (LiCl) and lithium oxide (Li₂O).
- Flame test: Lithium salts produce a characteristic crimson flame color when heated. This is due to the excitation of the single valence electron to higher energy levels, followed by the emission of light as the electron returns to its ground state.
The ease with which lithium loses its valence electron is a direct consequence of its electron configuration and low ionization energy.
Lithium's Position in the Periodic Table and its Electron Configuration
Lithium's position in the periodic table – Group 1 (alkali metals) – reflects its electron configuration. All alkali metals have a single valence electron in their outermost s orbital, leading to similar chemical properties, including high reactivity and the formation of +1 ions. The trend in reactivity within the alkali metals is linked to the increasing atomic size and decreasing ionization energy as you move down the group.
Beyond the Basics: Excited States and Spectroscopic Analysis
While the 1s²2s¹ configuration represents lithium's ground state (lowest energy state), electrons can absorb energy and transition to higher energy levels, known as excited states. These excited states are not stable and the electron will quickly return to the ground state, emitting energy in the form of light. This emission of light forms the basis of spectroscopic analysis, a technique used to identify elements based on their characteristic emission spectra. The characteristic crimson color observed in lithium's flame test is a result of electronic transitions between energy levels, ultimately linked to its electron configuration.
Conclusion: The Significance of Lithium's Electron Configuration
The electron configuration of lithium, 1s²2s¹, is fundamental to understanding its chemical and physical behavior. Its single valence electron is responsible for its reactivity, the formation of ionic compounds, and its spectroscopic characteristics. By understanding the principles of electron configuration, quantum numbers, and orbital diagrams, we gain a profound appreciation for how the arrangement of electrons dictates the properties of this essential element and its role in various applications, from batteries to medicine. Further exploration into its properties will uncover more facets of this remarkably versatile element.
Latest Posts
Latest Posts
-
Why Is Mol The Abbreviation To Mle
Mar 31, 2025
-
What Is The Correct General Equation For Cellular Respiration
Mar 31, 2025
-
Which Element Is The Least Reactive
Mar 31, 2025
-
Which One Is Good Insulator Metals Metalloids Or Nonmetals
Mar 31, 2025
-
Why Are Covalent Compounds Not Conductive
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
