What Is The Horizontal Row In The Periodic Table Called
Muz Play
Mar 25, 2025 · 7 min read
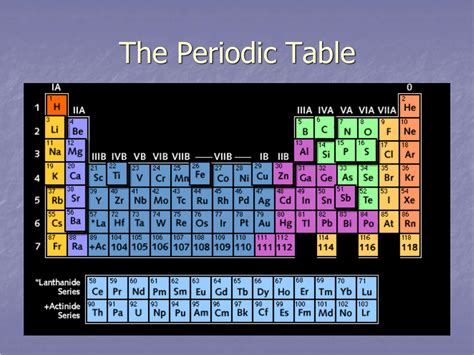
Table of Contents
- What Is The Horizontal Row In The Periodic Table Called
- Table of Contents
- What is the Horizontal Row in the Periodic Table Called? A Deep Dive into Periods and Their Significance
- Understanding Periods: A Horizontal Journey Through Atomic Structure
- Period 1: The Simplest Beginning
- Period 2 and 3: Expanding the Electron Shells
- Periods 4 and 5: The Introduction of d-Block Elements
- Periods 6 and 7: The Complexity of f-Block Elements
- Periodic Trends: Observing Patterns Across the Periods
- Atomic Radius: A Decreasing Trend
- Ionization Energy: An Increasing Trend
- Electronegativity: A Growing Attraction
- Electron Affinity: A Complex Relationship
- The Significance of Periods in Chemical Reactivity
- Predicting Chemical Behavior Based on Periodicity
- Periods and the Modern Periodic Table: A Continuous Refinement
- Connecting Periods to Groups and Blocks
- Beyond the Basic Trends: A Deeper Look
- Conclusion: Periods - The Foundation of Chemical Understanding
- Latest Posts
- Latest Posts
- Related Post
What is the Horizontal Row in the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many are familiar with its overall structure, a common question arises: what is the horizontal row in the periodic table called? The answer is a period. This seemingly simple term belies the wealth of information embedded within each row and its crucial role in understanding the behavior of elements. This article will delve deep into the concept of periods, exploring their significance, the trends observed within them, and how they relate to the broader organization of the periodic table.
Understanding Periods: A Horizontal Journey Through Atomic Structure
Each period in the periodic table represents a principal energy level or electron shell. As you move across a period from left to right, the atomic number increases, signifying an increase in the number of protons and electrons. Crucially, the elements within a period share the same highest occupied principal energy level. This means their outermost electrons occupy the same shell, significantly influencing their chemical properties.
Period 1: The Simplest Beginning
The first period, the shortest of all, contains only two elements: hydrogen (H) and helium (He). They both fill the first principal energy level (n=1), which can only accommodate a maximum of two electrons. This limited capacity shapes the chemical behavior of these elements, making them fundamentally different from those in subsequent periods.
Period 2 and 3: Expanding the Electron Shells
Periods 2 and 3 demonstrate a significant expansion in the number of elements. They respectively fill the second (n=2) and third (n=3) principal energy levels, which accommodate more electrons. The increase in the number of electrons and the subsequent filling of subshells lead to a broader range of chemical properties within these periods. The filling of the s and p subshells within these periods leads to a gradual transition in properties, from highly reactive metals on the left to less reactive nonmetals on the right.
Periods 4 and 5: The Introduction of d-Block Elements
Periods 4 and 5 are longer than the previous periods due to the introduction of the d subshell. The d orbitals are added to the energy level, resulting in a block of transition metals in the middle of these periods. These transition metals exhibit a variety of oxidation states and often form colored compounds, characteristics stemming from their partially filled d orbitals. The increase in electron shielding from the inner electron shells also affects atomic size and ionization energy within these periods.
Periods 6 and 7: The Complexity of f-Block Elements
Periods 6 and 7 are the longest periods, incorporating the f subshell. The f block elements, known as the lanthanides (period 6) and actinides (period 7), are placed separately at the bottom of the periodic table to maintain the table's manageable width. These elements exhibit complex electronic configurations and often show similar chemical properties within their respective series. The inclusion of the f orbitals significantly increases the number of electrons within the outermost shells impacting their chemical reactivity and properties.
Periodic Trends: Observing Patterns Across the Periods
The arrangement of elements within periods allows us to observe predictable trends in their properties. These trends are vital for understanding the reactivity and behavior of elements. Key periodic trends observed across periods include:
Atomic Radius: A Decreasing Trend
As you move across a period from left to right, the atomic radius generally decreases. This is because the number of protons increases, resulting in a stronger positive charge in the nucleus. This stronger positive charge pulls the electrons closer to the nucleus, thus reducing the atomic size. However, slight variations can be observed due to electron-electron repulsion and electron shielding effects.
Ionization Energy: An Increasing Trend
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This is a direct consequence of the increasing nuclear charge. The tighter hold of the nucleus on the electrons makes it increasingly difficult to remove an electron, therefore increasing the ionization energy. Exceptions to this trend can occur due to electronic configurations.
Electronegativity: A Growing Attraction
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. As the nuclear charge increases, the atom's ability to attract electrons in a bond also increases, leading to higher electronegativity. The noble gases are generally exceptions as they have full electron shells, resulting in low electronegativity.
Electron Affinity: A Complex Relationship
Electron affinity, the energy change associated with adding an electron to a neutral atom, shows a less straightforward trend across periods. While there is a general tendency for electron affinity to increase across a period, variations can arise due to electronic configurations and electron-electron repulsions.
The Significance of Periods in Chemical Reactivity
The period number directly relates to the valence electrons, the electrons in the outermost shell. These valence electrons are primarily responsible for the chemical reactivity of an element. Elements within the same period have different numbers of valence electrons but share the same highest occupied principal energy level. This determines the number of chemical bonds they can form and the type of compounds they can produce. For instance, elements in the first period (hydrogen and helium) have only one electron shell and have limited bonding capabilities. The elements in the second period (lithium to neon) have two electron shells, impacting their bonding behaviors, and so on.
Predicting Chemical Behavior Based on Periodicity
Understanding the periodic trends and the significance of valence electrons allows us to predict the chemical behavior of elements. For example, alkali metals (Group 1) in any period are highly reactive due to their single valence electron, readily participating in ionic bonding. Halogens (Group 17) are also highly reactive due to their seven valence electrons, having a strong tendency to gain an electron to achieve a stable octet configuration. The noble gases (Group 18) are inert due to their full valence shells.
Periods and the Modern Periodic Table: A Continuous Refinement
The modern periodic table, while appearing straightforward, is the product of centuries of scientific discovery. The concept of periods evolved alongside the understanding of atomic structure and the development of quantum mechanics. Initially, the organization was primarily based on atomic weight, but the discovery of atomic number and electron configuration led to a more accurate and insightful arrangement. The identification of periodic trends further solidified the importance of arranging elements according to periods.
Connecting Periods to Groups and Blocks
Periods are interwoven with other organizational features of the periodic table, namely groups (vertical columns) and blocks (regions associated with specific subshells). Elements within the same group share similar chemical properties due to the same number of valence electrons. However, the elements within a group will have varying principal energy levels as we move down the group. The blocks — s, p, d, and f — provide additional insight into the electron configuration and the subsequent properties.
Beyond the Basic Trends: A Deeper Look
While basic periodic trends provide a good starting point, the reality is more nuanced. The influence of electron-electron repulsion, shielding effects, and relativistic effects on heavier elements can create deviations from these trends. Understanding these subtle nuances requires a more in-depth analysis of atomic structure and quantum mechanics.
Conclusion: Periods - The Foundation of Chemical Understanding
The horizontal row in the periodic table, the period, is far more than a simple line of elements. It represents a fundamental layer in the organization of matter, reflecting the structure of atoms and directly impacting their properties. Understanding the concept of periods, the trends observed within them, and their connection to other organizational features of the periodic table is crucial for mastering chemistry. From predicting chemical reactivity to understanding the behavior of elements in various chemical reactions, periods serve as a cornerstone of our understanding of the chemical world. By grasping the intricacies of periods, we unlock a deeper appreciation for the elegance and power of the periodic table itself. It is a tool that not only organizes elements but also provides invaluable insights into the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
Is Salt A Pure Substance Or A Mixture
Mar 28, 2025
-
What Is The Subscript In Chemistry
Mar 28, 2025
-
What Is The Ph Of Salt
Mar 28, 2025
-
Activity Series Of Metals And Non Metals
Mar 28, 2025
-
How Many Unpaired Electrons Does Carbon Have
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Horizontal Row In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
