When Dissolved In Water An Arrhenius Base Yields
Muz Play
Mar 18, 2025 · 5 min read
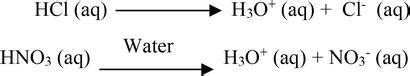
Table of Contents
- When Dissolved In Water An Arrhenius Base Yields
- Table of Contents
- When Dissolved in Water, an Arrhenius Base Yields: A Deep Dive into Base Chemistry
- The Arrhenius Definition of a Base
- The Role of Hydroxide Ions
- What Happens When an Arrhenius Base Dissolves in Water?
- Examples of Arrhenius Base Dissociation:
- Strong vs. Weak Arrhenius Bases
- Strong Arrhenius Bases
- Weak Arrhenius Bases
- The Impact on pH
- Practical Applications
- Industrial Applications:
- Everyday Applications:
- Beyond the Arrhenius Definition
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
When Dissolved in Water, an Arrhenius Base Yields: A Deep Dive into Base Chemistry
Understanding the behavior of bases in aqueous solutions is fundamental to chemistry. This article delves into the Arrhenius definition of a base, exploring what happens when an Arrhenius base dissolves in water and the implications of this process. We'll cover the concepts of hydroxide ions, pH, strong versus weak bases, and the practical applications of this knowledge.
The Arrhenius Definition of a Base
The Arrhenius definition, while somewhat limited compared to later, broader definitions like Brønsted-Lowry and Lewis, provides a crucial foundation for understanding basic chemistry. According to Arrhenius, a base is a substance that, when dissolved in water, increases the concentration of hydroxide ions (OH⁻). This increase in hydroxide ions is the key characteristic that defines an Arrhenius base. It's this direct production of OH⁻ ions that differentiates Arrhenius bases from other types of bases.
The Role of Hydroxide Ions
Hydroxide ions are negatively charged ions composed of one oxygen atom and one hydrogen atom. They are highly reactive and play a pivotal role in many chemical reactions. The presence of hydroxide ions in a solution is what makes the solution alkaline or basic. The more hydroxide ions present, the more basic (or alkaline) the solution becomes.
What Happens When an Arrhenius Base Dissolves in Water?
When an Arrhenius base dissolves in water, it undergoes dissociation. This means that the base molecule breaks apart into its constituent ions. For many common Arrhenius bases, this dissociation involves the release of hydroxide ions (OH⁻) into the solution.
Examples of Arrhenius Base Dissociation:
Let's illustrate this with some examples:
-
Sodium hydroxide (NaOH): A strong Arrhenius base. When dissolved in water, it completely dissociates into sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH(s) → Na⁺(aq) + OH⁻(aq) -
Potassium hydroxide (KOH): Another strong Arrhenius base. Similar to NaOH, it completely dissociates in water:
KOH(s) → K⁺(aq) + OH⁻(aq) -
Calcium hydroxide (Ca(OH)₂): This is also a base, but it produces two hydroxide ions per formula unit:
Ca(OH)₂(s) → Ca²⁺(aq) + 2OH⁻(aq)
These equations show that the base molecules break apart, releasing hydroxide ions directly into the aqueous solution. The presence of these hydroxide ions is directly responsible for the increase in the solution's pH.
Strong vs. Weak Arrhenius Bases
Arrhenius bases are categorized as either strong or weak based on their degree of dissociation in water.
Strong Arrhenius Bases
Strong Arrhenius bases completely dissociate into their ions when dissolved in water. This means that essentially 100% of the base molecules break apart, releasing all their hydroxide ions into the solution. Examples include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)₂)
- Barium hydroxide (Ba(OH)₂)
- Lithium hydroxide (LiOH)
Weak Arrhenius Bases
Weak Arrhenius bases only partially dissociate in water. This means that only a small percentage of the base molecules break apart, releasing a limited number of hydroxide ions. The majority of the base remains in its molecular form. Examples include:
-
Ammonia (NH₃): Ammonia reacts with water to form a small amount of ammonium ions (NH₄⁺) and hydroxide ions (OH⁻):
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)Note the equilibrium arrow (⇌), indicating that the reaction is reversible and doesn't go to completion.
-
Many metal hydroxides: While many metal hydroxides are considered Arrhenius bases, many are only slightly soluble in water, meaning that even if they were to completely dissociate, the limited amount dissolved would result in a relatively low concentration of hydroxide ions.
The Impact on pH
The concentration of hydroxide ions (OH⁻) directly affects the pH of a solution. pH is a measure of the acidity or basicity of a solution, with a scale ranging from 0 to 14. A pH of 7 is neutral, a pH below 7 is acidic, and a pH above 7 is basic (or alkaline).
The relationship between pH and pOH (the negative logarithm of the hydroxide ion concentration) is given by:
pH + pOH = 14
The higher the concentration of hydroxide ions, the higher the pOH and the higher the pH (more basic). Strong Arrhenius bases, due to their complete dissociation, produce significantly higher hydroxide ion concentrations and thus higher pH values compared to weak Arrhenius bases.
Practical Applications
The properties of Arrhenius bases and their behavior in water have numerous practical applications:
Industrial Applications:
- Soap and detergent production: Many soaps and detergents are made using strong Arrhenius bases like NaOH or KOH.
- Chemical synthesis: Arrhenius bases are used extensively as catalysts and reactants in many chemical processes.
- Water treatment: Bases are used to adjust the pH of water in various industrial and municipal applications.
Everyday Applications:
- Drain cleaners: Many drain cleaners contain strong Arrhenius bases that help dissolve organic matter that clogs drains.
- Antacids: Some antacids contain weak Arrhenius bases that neutralize stomach acid.
Beyond the Arrhenius Definition
While the Arrhenius definition of a base is useful for understanding many common bases, it has limitations. It only applies to aqueous solutions and doesn't account for bases that don't contain hydroxide ions. More comprehensive definitions, such as the Brønsted-Lowry and Lewis definitions, provide a broader understanding of base chemistry. However, understanding the Arrhenius definition remains a crucial stepping stone in grasping these more advanced concepts.
Conclusion
When an Arrhenius base dissolves in water, it increases the concentration of hydroxide ions (OH⁻). This increase in hydroxide ions is the defining characteristic of an Arrhenius base. The extent of this increase depends on whether the base is strong or weak. Strong bases completely dissociate, while weak bases only partially dissociate. This fundamental process has wide-ranging implications in various chemical processes and practical applications. Understanding the behavior of Arrhenius bases in water is essential for anyone studying chemistry or working in related fields. This knowledge forms the groundwork for a deeper comprehension of acid-base chemistry and its importance across diverse disciplines. The implications of this seemingly simple reaction are far-reaching and fundamental to a vast array of scientific and industrial processes. From the everyday use of antacids to the complex chemical reactions within industrial settings, the principles discussed here are consistently at play. Further exploration into Brønsted-Lowry and Lewis theories can extend this understanding even further, building upon the solid foundation established by the Arrhenius definition.
Latest Posts
Latest Posts
-
Do Acids Gain Or Lose Hydrogen Ions
Mar 19, 2025
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
-
How Is The Air Volume Affected By Temperature
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about When Dissolved In Water An Arrhenius Base Yields . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
