Which Nuclear Emission Has The Greatest Penetrating Power
Muz Play
Mar 20, 2025 · 5 min read
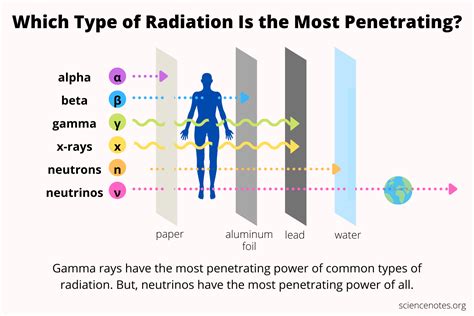
Table of Contents
Which Nuclear Emission Has the Greatest Penetrating Power? Understanding Alpha, Beta, and Gamma Radiation
The world of nuclear physics can seem daunting, filled with complex terminology and potentially dangerous phenomena. One key concept to grasp is the penetrating power of different types of nuclear emissions. Understanding this is crucial not only for scientific accuracy but also for safety protocols in handling radioactive materials and understanding the effects of radiation exposure. This article delves deep into the penetrating abilities of alpha, beta, and gamma radiation, exploring their properties and the mechanisms behind their varying interactions with matter.
Understanding the Three Main Types of Nuclear Emissions
Nuclear emissions are energetic particles or rays released during radioactive decay. The three primary types—alpha, beta, and gamma—differ significantly in their mass, charge, and energy, directly influencing their penetrating power.
Alpha Particles (α)
Alpha particles are relatively large and heavy, composed of two protons and two neutrons—essentially a helium nucleus. This gives them a positive charge of +2. Because of their size and mass, alpha particles are highly ionizing. This means they readily interact with matter, losing their energy quickly by colliding with and ionizing atoms in their path.
-
Penetration: Due to their high ionizing power, alpha particles have a very short range. A sheet of paper or even the outer layer of skin can effectively stop them. They are not considered a significant external radiation hazard, but internal exposure from inhaled or ingested alpha-emitting isotopes is extremely dangerous due to the intense localized damage.
-
Ionizing Power: Extremely high. Their large size and charge lead to frequent interactions with atoms.
-
Velocity: Relatively slow compared to beta and gamma radiation.
Beta Particles (β)
Beta particles are much smaller and lighter than alpha particles. They are high-energy electrons (β⁻) or positrons (β⁺), which are the antimatter counterparts of electrons (possessing the same mass but opposite charge). Beta particles carry a single negative or positive charge.
-
Penetration: Beta particles have a greater penetrating power than alpha particles. They can penetrate several millimeters of aluminum or a few centimeters of tissue. Thicker materials like wood or plastic are needed for effective shielding.
-
Ionizing Power: Moderately high. Their smaller size compared to alpha particles results in fewer interactions with matter, thus a longer range.
-
Velocity: Much faster than alpha particles.
Gamma Rays (γ)
Gamma rays are high-energy electromagnetic radiation, similar to X-rays but with even shorter wavelengths and higher energies. They are uncharged and massless.
-
Penetration: Gamma rays possess the highest penetrating power of the three types of nuclear emission. They can travel considerable distances through air and penetrate thick materials. Shielding requires dense materials like lead or concrete.
-
Ionizing Power: Relatively low. They interact with matter less frequently than alpha or beta particles. Their energy is transferred through interactions with the atomic nuclei or electrons of the material they pass through. This may cause ionization, but it's less frequent than with charged particles.
-
Velocity: The speed of light.
Comparing Penetrating Power: A Summary Table
To summarize the differences in penetrating power:
| Radiation Type | Charge | Mass | Penetrating Power | Shielding Material | Ionizing Power |
|---|---|---|---|---|---|
| Alpha (α) | +2 | High | Very low | Paper, skin | Very high |
| Beta (β) | -1 or +1 | Low | Moderate | Aluminum, wood | Moderate |
| Gamma (γ) | 0 | 0 | High | Lead, concrete | Low |
The Mechanisms Behind Penetrating Power
The penetrating power of each radiation type is directly linked to its interaction with matter. Charged particles (alpha and beta) primarily lose energy through ionization, stripping electrons from atoms in the material they traverse. The more massive and highly charged alpha particles interact more frequently, leading to a shorter range. Beta particles, being lighter and less charged, interact less frequently, resulting in a longer range.
Gamma rays, being uncharged, interact primarily through photoelectric absorption, Compton scattering, and pair production. These interactions are less frequent than ionization, allowing gamma rays to penetrate much further before losing their energy.
-
Photoelectric absorption: A gamma ray interacts with an inner electron of an atom, transferring all its energy and ejecting the electron. This is most likely to occur with low-energy gamma rays and high-atomic number materials.
-
Compton scattering: A gamma ray interacts with an outer electron of an atom, transferring part of its energy and scattering in a different direction. The scattered photon has lower energy and the electron recoils. This interaction dominates for medium energy gamma rays and is less dependent on material atomic number.
-
Pair production: A high-energy gamma ray interacts with the electric field near an atomic nucleus, converting its energy into an electron-positron pair. This process only occurs when the gamma ray energy is greater than 1.02 MeV (the combined rest mass energy of an electron and a positron).
Applications and Implications
The varying penetrating power of these emissions has significant implications across various fields:
-
Radiation Therapy: Alpha and beta emitters are used in targeted radiotherapy due to their limited range, delivering high doses of radiation to cancerous cells while minimizing damage to surrounding healthy tissue. Gamma rays, on the other hand, are used in external beam radiotherapy where deeper penetration is required.
-
Nuclear Medicine: Radioactive isotopes are employed for diagnostic imaging. The choice of isotope depends on the target tissue and the required penetration depth.
-
Industrial Applications: Gamma radiation is used for sterilization and quality control in industrial processes due to its high penetration power.
-
Radiation Safety: Understanding the penetrating power of different emissions is critical for designing appropriate shielding and safety protocols to protect workers and the public from radiation exposure. Shielding materials are chosen based on the type and energy of the radiation being shielded.
Conclusion: Gamma Rays Reign Supreme
In conclusion, gamma rays possess the greatest penetrating power among alpha, beta, and gamma radiation. This is a direct consequence of their lack of charge and mass, leading to less frequent interactions with matter compared to charged particles like alpha and beta particles. While alpha particles cause intense localized damage due to high ionization, gamma rays can penetrate much further, posing a different, albeit potentially equally dangerous type of radiation hazard due to their far-reaching effects. Understanding these differences is paramount for safety, effective use in various applications, and responsible management of radioactive materials. Further research into radiation shielding and detection techniques continues to evolve, improving safety standards and expanding the applications of nuclear technology.
Latest Posts
Latest Posts
-
How To Calculate Kb From Ka
Mar 20, 2025
-
Reactions Of Benzene And Substituted Benzenes
Mar 20, 2025
-
Kirby Bauer Method Of Antibiotic Sensitivity Testing
Mar 20, 2025
-
Are Covalent Bonds Soluble In Water
Mar 20, 2025
-
What Are The Products Of The Neutralization Reaction
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which Nuclear Emission Has The Greatest Penetrating Power . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
