A Polar Covalent Bond Is Created When
Muz Play
Mar 28, 2025 · 7 min read
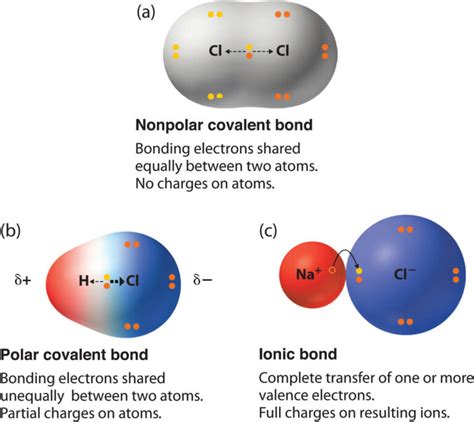
Table of Contents
A Polar Covalent Bond is Created When… Unequal Sharing Leads to a Dipolar Moment
A polar covalent bond, a fundamental concept in chemistry, forms the backbone of many molecules responsible for life's processes and the properties of countless materials. Understanding its formation is key to grasping the behavior of matter at the molecular level. This comprehensive guide will delve into the specifics of polar covalent bond formation, exploring the conditions necessary for its creation, its characteristics, and its implications in various chemical and biological systems.
Understanding the Building Blocks: Atoms and Electrons
Before diving into polar covalent bonds, let's refresh our understanding of atoms and their electrons. Atoms, the fundamental units of matter, comprise a nucleus containing protons and neutrons, surrounded by electrons orbiting in energy levels or shells. These electrons, particularly those in the outermost shell (valence electrons), play a crucial role in chemical bonding. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (a full outermost shell), a principle known as the octet rule.
The Role of Electronegativity
Electronegativity is a crucial factor determining the nature of a chemical bond. It measures an atom's ability to attract electrons in a chemical bond. Atoms with high electronegativity strongly attract electrons, while those with low electronegativity attract electrons weakly. This difference in electronegativity is the key to understanding the formation of polar covalent bonds. The Pauling scale is commonly used to quantify electronegativity, with fluorine (F) having the highest value (4.0).
The Formation of a Covalent Bond: Sharing is Caring
A covalent bond forms when two atoms share one or more pairs of electrons to achieve a stable electron configuration. This sharing allows both atoms to "fill" their valence shells, satisfying the octet rule (or duet rule for hydrogen). Consider the formation of a hydrogen molecule (H₂): each hydrogen atom has one electron, and by sharing their electrons, both atoms achieve a stable configuration of two electrons, effectively completing their first energy shell.
Nonpolar Covalent Bonds: Equal Sharing
In a nonpolar covalent bond, the sharing of electrons between atoms is essentially equal. This occurs when the two atoms have similar or identical electronegativities. A classic example is the bond between two hydrogen atoms in H₂, or the bond between two carbon atoms in a diamond crystal. Because the electrons are shared equally, there's no significant charge separation across the bond.
The Birth of a Polar Covalent Bond: Unequal Sharing
A polar covalent bond is formed when two atoms with significantly different electronegativities share electrons. Because of this difference, the atom with the higher electronegativity attracts the shared electrons more strongly. This unequal sharing creates a dipole moment, meaning a separation of charge across the bond. The atom with the higher electronegativity gains a partial negative charge (δ-), while the atom with the lower electronegativity gains a partial positive charge (δ+).
Visualizing the Dipole Moment
Imagine a tug-of-war between two atoms with different strengths. The stronger atom (higher electronegativity) pulls the electrons closer to itself, creating an imbalance in charge distribution. This imbalance is represented by the dipole moment, often depicted with an arrow pointing towards the more electronegative atom. The arrowhead indicates the partial negative charge (δ-), and a cross indicates the partial positive charge (δ+).
Examples of Polar Covalent Bonds
Many common molecules contain polar covalent bonds. Let's explore some illustrative examples:
-
Water (H₂O): Oxygen (O) is significantly more electronegative than hydrogen (H). The oxygen atom attracts the shared electrons more strongly, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens. This polarity makes water a highly effective solvent and contributes to its unique properties.
-
Hydrogen Fluoride (HF): Fluorine (F) is the most electronegative element. The bond between hydrogen and fluorine is highly polar, with a large dipole moment. This strong polarity leads to HF's high boiling point and its ability to act as a strong acid.
-
Ammonia (NH₃): Nitrogen (N) is more electronegative than hydrogen (H). The N-H bonds are polar, leading to the overall polarity of the ammonia molecule. This polarity affects ammonia's solubility in water and its ability to act as a weak base.
-
Carbon Monoxide (CO): Although carbon and oxygen are both non-metals, oxygen's higher electronegativity leads to a polar covalent bond with a significant dipole moment. This polarity impacts CO's reactivity and its toxicity.
The Magnitude of Polarity: The Electronegativity Difference
The degree of polarity in a covalent bond is directly related to the difference in electronegativity between the two bonded atoms. A larger electronegativity difference translates to a more polar bond. While there's no strict cutoff, a difference of approximately 0.4 or greater is generally considered to indicate a polar covalent bond. However, it's important to consider the molecular geometry; even with polar bonds, a symmetrical molecule might have a net dipole moment of zero.
Implications of Polarity: Solubility, Boiling Point, and More
The polarity of covalent bonds significantly influences the physical and chemical properties of molecules.
Solubility: "Like Dissolves Like"
Polar molecules tend to dissolve well in polar solvents, such as water, while nonpolar molecules dissolve well in nonpolar solvents, such as oil. This is often summarized as "like dissolves like". The polar nature of water allows it to interact effectively with other polar molecules through dipole-dipole interactions and hydrogen bonding.
Boiling Point: Stronger Intermolecular Forces
Polar molecules exhibit stronger intermolecular forces than nonpolar molecules. These stronger forces (dipole-dipole interactions, hydrogen bonding) require more energy to overcome, resulting in higher boiling points for polar substances compared to their nonpolar counterparts. For example, water (a polar molecule) has a significantly higher boiling point than methane (a nonpolar molecule).
Reactivity: Influence on Chemical Reactions
The polarity of a molecule influences its reactivity. Polar molecules participate readily in reactions involving polar reagents, while nonpolar molecules react more readily with nonpolar reagents. The partial charges in polar molecules create sites for electrostatic interactions, favoring certain reaction pathways.
Distinguishing Polar Covalent from Other Bonds
It's crucial to differentiate polar covalent bonds from other types of chemical bonds:
-
Ionic Bonds: In ionic bonds, electrons are completely transferred from one atom to another, creating ions with full charges (cations and anions). The electrostatic attraction between these oppositely charged ions forms the ionic bond. The electronegativity difference between the atoms is very large (typically greater than 1.7).
-
Nonpolar Covalent Bonds: As discussed earlier, nonpolar covalent bonds involve the equal sharing of electrons between atoms with similar electronegativities. There is no significant charge separation.
Advanced Concepts and Applications
The understanding of polar covalent bonds extends beyond basic chemistry and finds applications in diverse fields:
-
Biochemistry: Polarity plays a vital role in protein folding, enzyme-substrate interactions, and the transport of molecules across cell membranes. Many biomolecules, like amino acids and sugars, contain polar functional groups that are critical for their biological functions.
-
Materials Science: The polarity of materials influences their properties, such as dielectric constant, surface tension, and adhesion. Understanding polarity helps in designing materials with specific desired characteristics.
-
Drug Design: The polarity of drug molecules affects their absorption, distribution, metabolism, and excretion (ADME) properties. Designing drugs with appropriate polarity is crucial for their effectiveness and safety.
-
Environmental Science: Polarity influences the behavior of pollutants in the environment. For example, polar pollutants tend to dissolve in water, while nonpolar pollutants may persist in soil or accumulate in organisms.
Conclusion: A Cornerstone of Chemistry
The formation of a polar covalent bond, driven by unequal electron sharing due to a difference in electronegativity, is a fundamental concept in chemistry. Understanding this concept allows us to predict and explain the properties of a vast range of molecules, from simple diatomic molecules to complex biomolecules. The polarity of molecules influences their physical properties, chemical reactivity, and biological functions, highlighting the significance of polar covalent bonds in our understanding of the natural world. From the water we drink to the proteins in our bodies, polar covalent bonds are ubiquitous, making their study essential for comprehending the molecular basis of life and matter.
Latest Posts
Latest Posts
-
Choose The Correct Names Of The Atoms Or Molecules
Mar 31, 2025
-
Integrals Of Even And Odd Functions
Mar 31, 2025
-
What Makes Something A Strong Acid
Mar 31, 2025
-
According To The Rules Of Osmosis A System Will
Mar 31, 2025
-
Where Are Chondrocytes And Osteocytes Located
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about A Polar Covalent Bond Is Created When . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
