Define Family In The Periodic Table
Muz Play
Mar 21, 2025 · 6 min read
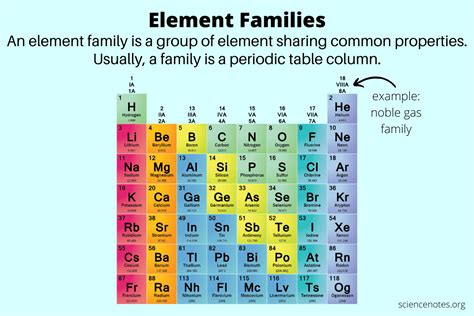
Table of Contents
Defining Family in the Periodic Table: Exploring Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. While the arrangement reveals trends and patterns, the concept of "family" takes on a special significance. In this context, "family" refers to groups or columns of elements, sharing similar outer electron configurations and, consequently, exhibiting analogous chemical behaviors. Understanding these families is crucial for predicting an element's reactivity, bonding patterns, and overall chemical characteristics. This article will delve deep into the definition of families in the periodic table, exploring their key characteristics, representative members, and applications.
The Significance of Outer Electrons: Valence Shells and Chemical Behavior
The defining feature of a family in the periodic table is the similarity in their valence electrons. These are the electrons located in the outermost shell, or valence shell, of an atom. Valence electrons are the primary players in chemical bonding – the forces that hold atoms together to form molecules and compounds. Elements within the same group possess the same number of valence electrons, leading to strikingly similar chemical behavior.
For example, elements in Group 1, the alkali metals (lithium, sodium, potassium, etc.), all have one valence electron. This single valence electron readily participates in chemical reactions, making alkali metals highly reactive and prone to losing that electron to achieve a stable electron configuration. Similarly, Group 17, the halogens (fluorine, chlorine, bromine, etc.), all have seven valence electrons. They readily gain one electron to achieve a stable octet (eight valence electrons), resulting in their high reactivity as well, although in a different way than alkali metals.
Electron Configuration and Periodicity: The Underlying Mechanism
The periodic nature of the table itself is a direct consequence of the filling of electron orbitals. As the atomic number increases, electrons fill orbitals in a predictable manner, following specific rules and principles (Aufbau principle, Hund's rule, Pauli exclusion principle). This filling pattern leads to the recurring similarities in valence electron configurations among elements in the same group. This underlying structure is the fundamental reason why elements in the same family exhibit similar properties.
Exploring Key Families in the Periodic Table: A Detailed Look
The periodic table is rich in families, each with its unique characteristics. Let’s examine some of the most prominent groups:
1. Group 1: Alkali Metals
- Defining Characteristic: One valence electron.
- Reactivity: Extremely reactive, readily losing their valence electron to form +1 ions. React violently with water.
- Physical Properties: Soft, silvery-white metals with low melting points and densities. Good conductors of electricity and heat.
- Representative Members: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
- Applications: Lithium in batteries; sodium in table salt (NaCl); potassium in fertilizers.
2. Group 2: Alkaline Earth Metals
- Defining Characteristic: Two valence electrons.
- Reactivity: Reactive, but less so than alkali metals. Lose two electrons to form +2 ions.
- Physical Properties: Harder, denser, and have higher melting points than alkali metals. Also good conductors of electricity and heat.
- Representative Members: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
- Applications: Magnesium in alloys; calcium in bones and teeth; strontium in fireworks.
3. Group 17: Halogens
- Defining Characteristic: Seven valence electrons.
- Reactivity: Highly reactive nonmetals. Readily gain one electron to form -1 ions, achieving a stable octet.
- Physical Properties: Diatomic molecules (exist as pairs: F₂, Cl₂, etc.) Vary in physical state from gas (fluorine, chlorine) to liquid (bromine) to solid (iodine).
- Representative Members: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
- Applications: Fluorine in toothpaste; chlorine in water purification; iodine in disinfectants.
4. Group 18: Noble Gases
- Defining Characteristic: Eight valence electrons (except helium, which has two).
- Reactivity: Extremely unreactive, often called inert gases. Their stable electron configuration makes them reluctant to participate in chemical reactions.
- Physical Properties: All are gases at room temperature. Colorless, odorless, and tasteless.
- Representative Members: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
- Applications: Helium in balloons; neon in signage; argon in welding.
5. Transition Metals: Groups 3-12
- Defining Characteristic: Variable valence electrons, often participating in multiple oxidation states. The filling of d orbitals contributes to their diverse properties.
- Reactivity: Reactivity varies significantly across the series.
- Physical Properties: Generally hard, dense, and have high melting points. Excellent conductors of electricity and heat. Many exhibit characteristic colors in solution.
- Representative Members: Iron (Fe), Copper (Cu), Gold (Au), Platinum (Pt).
- Applications: Numerous applications in various industries due to their strength, conductivity, and catalytic properties.
6. Lanthanides and Actinides: The f-block elements
- Defining Characteristic: These elements involve the filling of the f orbitals.
- Reactivity: Their reactivity varies, with some showing high reactivity and others exhibiting relatively low reactivity.
- Physical Properties: Most are silvery-white metals. The actinides are radioactive.
- Representative Members: Lanthanum (La), Cerium (Ce), Uranium (U), Plutonium (Pu).
- Applications: Various applications, including in lighting, magnets, and nuclear technology.
Beyond the Simple Groups: Understanding Trends and Exceptions
While the grouping based on valence electrons provides a powerful framework, understanding the periodic table requires acknowledging nuances and exceptions. Trends in properties are often observed within groups and across periods (rows), but there can be deviations due to factors like:
- Effective Nuclear Charge: The net positive charge experienced by an electron, influenced by shielding effects from inner electrons.
- Atomic Size: The size of an atom, which impacts its interactions with other atoms.
- Electronegativity: The tendency of an atom to attract electrons towards itself in a chemical bond.
- Ionization Energy: The energy required to remove an electron from an atom.
These factors can subtly alter the behavior of certain elements within a group, leading to variations from the expected trends. For instance, some heavier elements in a group may exhibit slightly different chemical behaviors due to relativistic effects (effects caused by electrons moving at very high speeds). Moreover, the transition metals display a much more complex array of oxidation states and reactivity patterns compared to the main group elements.
Applications and Importance of Understanding Family Relationships
Knowing the families of elements is crucial for a variety of applications:
- Predicting Chemical Reactions: The similarity in valence electrons allows us to predict how elements in the same family will react with other substances.
- Material Science: Understanding the properties of elements allows us to design new materials with specific characteristics.
- Industrial Processes: Many industrial processes rely on the unique properties of specific elements and their families.
- Biological Systems: The role of essential elements (like calcium, potassium, sodium) in biological systems is directly linked to their chemical properties and family relationships.
- Environmental Science: Understanding the chemical behavior of elements is crucial for understanding environmental issues like pollution and remediation.
Conclusion: A Deeper Appreciation of the Periodic Table
The concept of "family" in the periodic table is a powerful tool for understanding the behavior of elements. By recognizing the relationship between electron configuration, valence electrons, and chemical properties, we can unlock a deep understanding of the periodic table and its implications in a variety of scientific and technological fields. While simple classifications based on groups are a good starting point, appreciating the subtle variations and exceptions within families leads to a more nuanced and comprehensive comprehension of the elegant structure and predictive power of the periodic table. Further exploration of individual families and their unique characteristics will continuously deepen our knowledge and unlock new applications for this indispensable tool of chemistry.
Latest Posts
Latest Posts
-
Atomic Number Equals The Number Of
Mar 22, 2025
-
Solids To Gases Row Or Column
Mar 22, 2025
-
What Color Is The Animal Cell
Mar 22, 2025
-
Is Silver A Metal Or Nonmetal Or Metalloid
Mar 22, 2025
-
The Higher The Boiling Point The More Polar
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Define Family In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
