Determining Rate Law From Experimental Data
Muz Play
Mar 17, 2025 · 6 min read
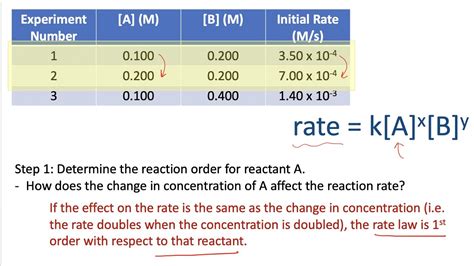
Table of Contents
Determining Rate Laws from Experimental Data: A Comprehensive Guide
Determining the rate law of a chemical reaction is crucial for understanding its mechanism and predicting its behavior under different conditions. Unlike the stoichiometry of a reaction, which is determined solely from the balanced chemical equation, the rate law must be experimentally determined. This article provides a comprehensive guide on how to determine rate laws from experimental data, covering various methods and considerations.
Understanding Rate Laws
Before delving into the methods, let's establish a foundational understanding of rate laws. A rate law expresses the relationship between the rate of a reaction and the concentrations of the reactants. A general form of a rate law is:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>...
Where:
- Rate: The rate of the reaction (often expressed as the change in concentration per unit time).
- k: The rate constant, a proportionality constant that depends on temperature and other factors.
- [A], [B], ...: The concentrations of reactants A, B, etc.
- m, n, ...: The reaction orders with respect to reactants A, B, etc. These are typically integers (0, 1, 2, etc.) but can sometimes be fractional or negative.
The overall reaction order is the sum of the individual reaction orders (m + n + ...).
Methods for Determining Rate Laws
Several methods exist for determining the rate law from experimental data. The most common are:
1. Method of Initial Rates
This method involves performing several experiments, each with different initial concentrations of reactants, and measuring the initial rate of the reaction. By comparing the initial rates, we can determine the reaction orders. Here's how it works:
Step 1: Gather Data
Conduct multiple experiments, varying the initial concentration of one reactant at a time while keeping others constant. Measure the initial rate of the reaction for each experiment. This typically involves measuring the concentration of a reactant or product over a short time interval at the beginning of the reaction.
Step 2: Determine Reaction Orders
Let's consider a simple reaction: A + B → Products
Compare two experiments where the concentration of reactant A is changed while the concentration of reactant B is held constant. The ratio of the rates is:
(Rate<sub>1</sub> / Rate<sub>2</sub>) = ([A]<sub>1</sub> / [A]<sub>2</sub>)<sup>m</sup>
By taking the logarithm of both sides, we can solve for 'm':
log(Rate<sub>1</sub> / Rate<sub>2</sub>) = m * log([A]<sub>1</sub> / [A]<sub>2</sub>)
Similarly, by comparing experiments where the concentration of B is changed while A is constant, we can determine 'n'.
Step 3: Determine the Rate Constant (k)
Once the reaction orders (m and n) are determined, substitute the values from any experiment into the rate law equation and solve for the rate constant k.
Example:
Let's say we have the following experimental data for the reaction A + B → Products:
| Experiment | [A] (M) | [B] (M) | Initial Rate (M/s) |
|---|---|---|---|
| 1 | 0.1 | 0.1 | 0.005 |
| 2 | 0.2 | 0.1 | 0.020 |
| 3 | 0.1 | 0.2 | 0.010 |
Comparing experiments 1 and 2:
(0.020/0.005) = (0.2/0.1)<sup>m</sup> => 4 = 2<sup>m</sup> => m = 2
Comparing experiments 1 and 3:
(0.010/0.005) = (0.2/0.1)<sup>n</sup> => 2 = 2<sup>n</sup> => n = 1
Therefore, the rate law is: Rate = k[A]<sup>2</sup>[B]
Using experiment 1 to find k:
0.005 = k(0.1)<sup>2</sup>(0.1) => k = 5 M<sup>-2</sup>s<sup>-1</sup>
2. Graphical Method
This method involves plotting the concentration of a reactant versus time and analyzing the shape of the curve. The order of the reaction with respect to that reactant can be determined from the shape of the graph.
- Zero-order reaction: A straight line with a negative slope.
- First-order reaction: A curved line that can be linearized by plotting ln[A] versus time (producing a straight line with a negative slope).
- Second-order reaction: A curved line that can be linearized by plotting 1/[A] versus time (producing a straight line with a positive slope).
This method is particularly useful when dealing with reactions involving only one reactant, or when isolating the effect of a single reactant. However, it is less suitable for complex reactions involving multiple reactants and higher-order kinetics.
3. Integrated Rate Laws
Integrated rate laws are mathematical expressions that relate the concentration of a reactant to time. They are derived from the differential rate laws (the rate laws we've discussed above) through integration. By fitting the experimental data to the integrated rate law equation for different orders, we can determine the reaction order.
For example:
- First-order reaction: ln[A]<sub>t</sub> = -kt + ln[A]<sub>0</sub> (linear plot of ln[A] vs. t)
- Second-order reaction: 1/[A]<sub>t</sub> = kt + 1/[A]<sub>0</sub> (linear plot of 1/[A] vs. t)
By plotting the appropriate function of concentration versus time and obtaining a linear relationship, we can determine the reaction order and calculate the rate constant from the slope of the line. This method is often used in conjunction with the graphical method, providing a more rigorous approach to determining the reaction order.
Considerations and Challenges
Determining rate laws from experimental data can be challenging. Several factors can influence the accuracy and reliability of the results:
- Experimental Error: Errors in measurements of concentration and time can significantly affect the calculated rate constants and reaction orders.
- Side Reactions: The presence of side reactions can complicate the analysis and lead to inaccurate rate laws.
- Non-ideal conditions: Deviations from ideal conditions (e.g., non-constant temperature, significant changes in volume) can introduce errors.
- Complex Reactions: Multi-step reactions with intermediate steps can lead to complex rate laws that are difficult to determine from experimental data alone.
- Reaction Mechanisms: The rate law does not directly reveal the reaction mechanism. While the rate law provides information about the rate-determining step, further investigation is often needed to elucidate the complete mechanism.
Advanced Techniques
For more complex reactions or when high accuracy is required, more sophisticated techniques may be necessary:
- Nonlinear regression: This statistical method can be used to fit the experimental data to various rate law models and determine the best fit. This is especially useful when dealing with non-integer reaction orders or complex rate laws.
- Computational methods: Computational chemistry and molecular dynamics simulations can provide theoretical insights into reaction mechanisms and rate constants, which can be compared to experimental data.
Conclusion
Determining the rate law from experimental data is a crucial step in understanding chemical kinetics. The methods described in this article, including the method of initial rates, the graphical method, and the use of integrated rate laws, provide powerful tools for this purpose. However, it's crucial to be aware of potential challenges and consider using advanced techniques when necessary to ensure accurate and reliable results. By carefully designing experiments, analyzing data, and understanding the limitations of each method, researchers can gain valuable insights into the behavior and mechanisms of chemical reactions. Remember that accuracy and precision in experimental design and data analysis are paramount in obtaining reliable rate laws. Careful attention to detail and the use of appropriate statistical methods will significantly enhance the validity and reliability of your findings.
Latest Posts
Latest Posts
-
The Ideal Osmotic Environment For An Animal Cell Is
Mar 17, 2025
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Determining Rate Law From Experimental Data . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
