Enantiomers Are Mirror Images Of Each Other.
Muz Play
Mar 17, 2025 · 5 min read
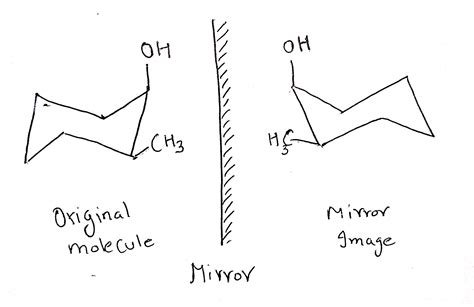
Table of Contents
Enantiomers: Mirror Images with Distinct Properties
Enantiomers are a fascinating topic in organic chemistry, captivating scientists and students alike. Their unique relationship – being mirror images of each other yet possessing distinct properties – highlights the intricate three-dimensional nature of molecules and their profound impact on biological systems. This article delves deep into the world of enantiomers, exploring their definition, properties, nomenclature, separation techniques, and biological significance.
Understanding Enantiomers: Beyond the Mirror Image
At first glance, enantiomers appear identical. They possess the same molecular formula and connectivity of atoms. However, a closer inspection reveals a crucial difference: they are non-superimposable mirror images of each other. This means that you cannot overlay one molecule onto its mirror image, no matter how you rotate it. Think of your left and right hands: they are mirror images, but you can't perfectly overlap them. This non-superimposability is the defining characteristic of enantiomers.
This chirality, or "handedness," arises from the presence of a stereocenter. A stereocenter is typically a carbon atom bonded to four different groups. This tetrahedral arrangement allows for two distinct spatial arrangements, leading to the formation of enantiomers.
The Importance of Three-Dimensional Structure
The seemingly subtle difference between enantiomers has profound consequences on their physical and chemical properties. While they have identical boiling points, melting points, and solubilities in achiral solvents (solvents that lack chirality), they exhibit different interactions with chiral environments. This includes interactions with other chiral molecules, polarized light, and enzymes.
Nomenclature of Enantiomers: R and S Configurations
The absolute configuration of enantiomers – describing the spatial arrangement of atoms around the stereocenter – is denoted using the Cahn-Ingold-Prelog (CIP) priority rules. This system assigns priorities to the four groups attached to the stereocenter based on atomic number. The molecule is then oriented so that the lowest priority group points away from the viewer. The remaining groups are examined in descending order of priority. If the order of priority is clockwise, the configuration is designated as R (rectus, Latin for right). If the order is counterclockwise, the configuration is S (sinister, Latin for left).
This systematic nomenclature is crucial for unambiguous identification and communication in chemistry and related fields.
Beyond R and S: Other Stereochemical Descriptors
While R/S notation is widely used, other descriptors also help characterize enantiomers. For example, the (+) and (-) notation refers to the direction in which a molecule rotates plane-polarized light. Enantiomers rotate plane-polarized light to equal but opposite extents – one clockwise ((+)-enantiomer, dextrorotatory) and the other counterclockwise ((-)-enantiomer, levorotatory). However, this notation doesn't directly relate to the R/S configuration. A molecule with an R configuration might be (+)-rotatory, while another with an R configuration could be (-)-rotatory, depending on the specific molecule and its structure.
Separating Enantiomers: Resolution Techniques
Since enantiomers have identical physical properties in achiral environments, separating them (a process known as resolution) presents a significant challenge. Several techniques are employed:
1. Chiral Chromatography: Separating Molecules Based on Chirality
Chiral chromatography utilizes a stationary phase containing a chiral selector. This chiral selector interacts differently with each enantiomer, leading to their separation as they pass through the column. The differing interactions are based on their different three-dimensional shapes and how they fit into the chiral environment of the stationary phase.
2. Diastereomer Formation: Converting Enantiomers into Separable Diastereomers
This method involves reacting the racemic mixture (a mixture containing equal amounts of both enantiomers) with a chiral resolving agent. This reaction creates a mixture of diastereomers – stereoisomers that are not mirror images. Diastereomers have different physical properties, making them separable using conventional techniques like crystallization or distillation.
3. Enzymatic Resolution: Harnessing the Power of Enzymes
Enzymes, being chiral themselves, selectively catalyze the reaction of one enantiomer, leaving the other unchanged. This selective reactivity can be exploited to separate enantiomers. This method is particularly useful in organic synthesis and pharmaceutical production.
Biological Significance of Enantiomers: A Tale of Two Molecules
The biological activity of many molecules is profoundly affected by their chirality. This is because biological systems, including enzymes and receptors, are themselves chiral. As a result, enantiomers often interact differently with biological targets, leading to vastly different pharmacological effects.
Enantiomer Selectivity in Drug Action
A classic example is thalidomide, a drug once used as a sedative. One enantiomer of thalidomide possesses therapeutic properties, while the other is teratogenic (causing birth defects). The tragic consequences of administering a racemic mixture highlighted the critical importance of considering chirality in drug development.
Chiral Molecules in Nature: From Amino Acids to Sugars
Nature predominantly uses one enantiomer of many biologically relevant molecules. For example, almost all naturally occurring amino acids are L-amino acids, and most sugars are D-sugars. This stereoselectivity is essential for the proper functioning of biological systems.
Enantiomers in Everyday Life: A Wider Perspective
The impact of enantiomers extends beyond the realm of pharmaceuticals. They play a vital role in various aspects of our daily lives:
-
Fragrances: The scent of a molecule can dramatically change depending on its chirality. For instance, one enantiomer of carvone smells like spearmint, while the other smells like caraway.
-
Flavors: Similar to fragrances, the taste of a compound can be drastically altered by its chirality.
-
Pesticides: The effectiveness of pesticides can be influenced by the chirality of their active ingredients. One enantiomer might be highly effective, while the other might be ineffective or even environmentally harmful.
Conclusion: A World of Chirality
Enantiomers, while seemingly subtle in their differences, reveal the intricate connection between molecular structure and function. Their distinct properties, particularly their interaction with chiral environments, have profound implications in various scientific fields, from medicine and pharmacology to environmental science and material science. Understanding the concept of enantiomers and the techniques for their separation is crucial for developing safe and effective drugs, designing environmentally friendly pesticides, and harnessing the power of nature's chiral molecules. The field continues to evolve, with ongoing research revealing the ever-expanding impact of chirality on our world. Further studies into the enantioselective synthesis and applications of chiral molecules will undoubtedly unlock new possibilities in various fields, shaping our future in innovative ways.
Latest Posts
Latest Posts
-
The Ideal Osmotic Environment For An Animal Cell Is
Mar 17, 2025
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Enantiomers Are Mirror Images Of Each Other. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
