Functional Groups Common To All Amino Acids
Muz Play
Mar 17, 2025 · 6 min read
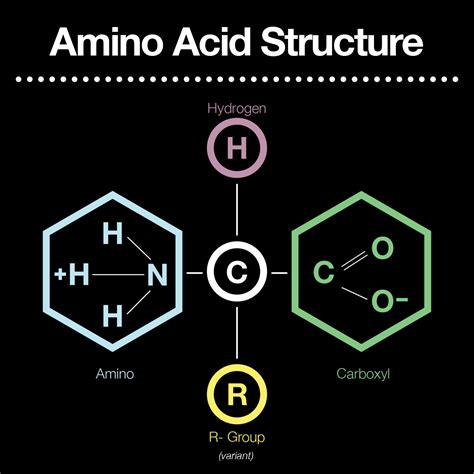
Table of Contents
Functional Groups Common to All Amino Acids: A Deep Dive
Amino acids, the fundamental building blocks of proteins, share a common structural framework characterized by specific functional groups. Understanding these functional groups is crucial to comprehending the diverse properties and functions of proteins. This article will delve into the common functional groups found in all amino acids, exploring their chemical properties and their impact on the overall behavior of amino acid molecules and the proteins they form.
The Core Structure: A Shared Foundation
Every amino acid, regardless of its specific side chain (R group), possesses a core structure comprising three key functional groups:
-
Amino Group (-NH₂): A basic group containing a nitrogen atom bonded to two hydrogen atoms. This group is responsible for the "amino" part of the amino acid name. Its basicity allows it to accept a proton (H⁺), becoming positively charged under acidic conditions. This characteristic is crucial for amino acid interactions within proteins and their overall three-dimensional structure.
-
Carboxylic Acid Group (-COOH): An acidic group containing a carboxyl group (-COOH). This group contributes the "acid" part of the name. Under basic conditions, it readily donates a proton (H⁺), forming a carboxylate anion (-COO⁻). This ability to donate a proton is key to its role in peptide bond formation and various enzymatic reactions.
-
α-Carbon: A central carbon atom (Cα) to which the amino group, carboxylic acid group, and the side chain (R group) are attached. The α-carbon is chiral in all amino acids except glycine, meaning it has four different groups attached. This chirality plays a significant role in the three-dimensional structure of proteins.
The Importance of the Core Structure
The presence of both an amino group and a carboxylic acid group in close proximity within the same molecule allows for a unique characteristic: zwitterionic form. At physiological pH (around 7.4), amino acids exist predominantly as zwitterions. In this form, the amino group accepts a proton becoming positively charged (+NH₃), while the carboxylic acid group donates a proton becoming negatively charged (-COO⁻). This internal charge balance profoundly influences the solubility and reactivity of amino acids.
The Variable Side Chain (R Group): A Spectrum of Diversity
While the core structure remains consistent across all amino acids, the side chain (R group) exhibits remarkable variability. This diversity is what gives rise to the 20 standard amino acids, each with unique chemical properties and functions. The R groups can be:
-
Nonpolar, aliphatic: These side chains are hydrophobic, meaning they repel water. Examples include alanine, valine, leucine, and isoleucine. These amino acids often cluster in the interior of proteins, away from the aqueous environment.
-
Aromatic: These side chains contain aromatic rings, imparting unique properties such as absorbance of ultraviolet light. Examples include phenylalanine, tyrosine, and tryptophan. Their aromatic nature contributes to protein structure and function through various interactions.
-
Polar, uncharged: These side chains are hydrophilic, meaning they are attracted to water. They contain functional groups capable of hydrogen bonding, such as hydroxyl (-OH) or amide (-CONH₂) groups. Examples include serine, threonine, asparagine, and glutamine. These residues often reside on the protein surface, interacting with the surrounding aqueous environment.
-
Positively charged (basic): These side chains contain positively charged nitrogen-containing functional groups at physiological pH. Examples include lysine, arginine, and histidine. These amino acids are often involved in electrostatic interactions within proteins and their interaction with other molecules.
-
Negatively charged (acidic): These side chains possess negatively charged carboxyl groups at physiological pH. Examples include aspartic acid and glutamic acid. These amino acids also participate in electrostatic interactions, contributing to the overall charge distribution and function of proteins.
The Impact of R Groups on Protein Structure and Function
The diversity of R groups is essential for the vast range of protein functions. The specific combination and arrangement of amino acids within a polypeptide chain dictates its three-dimensional structure, which in turn determines its function. Hydrophobic interactions, hydrogen bonds, disulfide bridges (formed between cysteine residues), and ionic interactions between amino acid side chains all contribute to this intricate process.
Peptide Bond Formation: Linking Amino Acids
The carboxylic acid group of one amino acid reacts with the amino group of another amino acid, forming a peptide bond (amide bond). This process releases a water molecule and creates a dipeptide. This process can continue, linking numerous amino acids together to form polypeptide chains, the precursors to proteins. The peptide bond is a crucial link that defines the primary structure of proteins.
The Partial Double Bond Character of the Peptide Bond
The peptide bond exhibits partial double-bond character due to resonance. This means that the electrons are delocalized, resulting in restricted rotation around the peptide bond. This rigidity plays a critical role in the secondary structure of proteins (α-helices and β-sheets).
Ionization States and pKa Values
The amino and carboxyl groups of amino acids can ionize depending on the surrounding pH. Each functional group has a characteristic pKa value, which represents the pH at which half of the molecules are ionized and half are not. The pKa values of the amino and carboxyl groups influence the charge of the amino acid at a specific pH, which further affects its interactions with other molecules and its role within a protein.
Isoelectric Point (pI)
The isoelectric point (pI) is the pH at which an amino acid carries a net charge of zero. This is an important characteristic as it affects the amino acid's behavior in electrophoresis and other separation techniques. The pI is calculated based on the pKa values of the ionizable groups.
Functional Groups and Protein Folding: A Complex Interplay
The various functional groups present in amino acids drive the process of protein folding. The interactions between these groups, including hydrogen bonds, hydrophobic interactions, ionic interactions, and disulfide bonds, dictate the three-dimensional structure of the protein. This intricate folding process is essential for the protein's biological activity. Misfolding can lead to the formation of non-functional proteins or even contribute to diseases like Alzheimer's and Parkinson's.
Post-Translational Modifications: Expanding Functional Diversity
After a protein is synthesized, its functional groups can undergo post-translational modifications. These modifications can alter the protein's properties and function, adding another layer of complexity to protein behavior. Examples of post-translational modifications include phosphorylation (addition of a phosphate group), glycosylation (addition of a carbohydrate), and acetylation (addition of an acetyl group). These modifications often involve the side chains of specific amino acids.
Conclusion: The Functional Group Perspective
The common functional groups – amino group, carboxylic acid group, and the α-carbon – form the foundation of all amino acids. However, the diverse nature of the side chains (R groups) allows for a remarkable spectrum of chemical properties. The interplay of these functional groups governs amino acid interactions, peptide bond formation, protein folding, and ultimately, the diverse functions of proteins within living organisms. A thorough understanding of these functional groups is therefore crucial for comprehending the intricacies of protein structure, function, and the broader field of biochemistry. Further research continues to reveal new and exciting aspects of amino acid chemistry and their impact on life processes. The exploration of these fundamental building blocks remains a vibrant and constantly evolving area of scientific inquiry.
Latest Posts
Latest Posts
-
The Ideal Osmotic Environment For An Animal Cell Is
Mar 17, 2025
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Functional Groups Common To All Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
