Group 2a Elements Are Also Called
Muz Play
Mar 27, 2025 · 5 min read
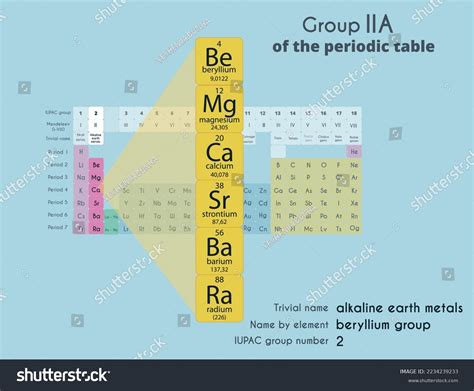
Table of Contents
Group 2A Elements: Also Known as Alkaline Earth Metals
Group 2A elements, also known as alkaline earth metals, are a fascinating group within the periodic table, exhibiting unique properties and playing crucial roles in various applications. Understanding their characteristics and applications is key to appreciating their significance in chemistry and beyond. This comprehensive article delves into the identity, properties, reactions, and uses of these essential elements.
Defining the Alkaline Earth Metals: A Closer Look at Group 2A
The alkaline earth metals comprise the six elements found in the second column of the periodic table: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They share similar electronic configurations, with two valence electrons in their outermost shell (ns²). This commonality dictates their chemical behavior and explains why they exhibit similar properties.
Distinguishing Features of Alkaline Earth Metals
Several key characteristics distinguish alkaline earth metals from other groups:
-
Metallic Character: They are all metals, exhibiting typical metallic properties such as high electrical and thermal conductivity, malleability, and ductility (though less so than alkali metals).
-
Reactivity: While less reactive than alkali metals, they are still reactive elements, readily losing their two valence electrons to form 2+ ions (M²⁺). This reactivity increases down the group as atomic size increases and ionization energy decreases.
-
Oxidation States: Their most common oxidation state is +2, reflecting the loss of those two valence electrons. Higher oxidation states are rare and less stable.
-
Physical Properties: These elements demonstrate a range of physical properties. For instance, beryllium is relatively hard and brittle, while magnesium is relatively light and strong. As you move down the group, density, melting point, and boiling point generally increase.
-
Electronic Configuration: As mentioned, the defining feature is their ns² electron configuration, where 'n' represents the principal quantum number. This commonality is the foundation of their similar chemical behavior.
Exploring the Chemistry of Alkaline Earth Metals: Reactions and Compounds
The chemical behavior of alkaline earth metals is primarily governed by their tendency to lose two electrons and form divalent cations (M²⁺). Let's explore some key reactions:
Reaction with Oxygen: Formation of Oxides
Alkaline earth metals readily react with oxygen (O₂) in air to form metal oxides. The reaction is generally more vigorous for the heavier elements:
2M(s) + O₂(g) → 2MO(s)
For example, magnesium burns brightly in air to produce magnesium oxide (MgO), commonly known as magnesia:
2Mg(s) + O₂(g) → 2MgO(s)
Reaction with Water: Hydroxide Formation
The reactivity with water increases down the group. Beryllium doesn't react with water, magnesium reacts slowly, and calcium, strontium, and barium react more readily, producing metal hydroxides and hydrogen gas:
M(s) + 2H₂O(l) → M(OH)₂(aq) + H₂(g)
Calcium's reaction with water is a good example:
Ca(s) + 2H₂O(l) → Ca(OH)₂(aq) + H₂(g)
Reaction with Acids: Salt Formation
Alkaline earth metals react with dilute acids (such as hydrochloric acid or sulfuric acid) to produce metal salts and hydrogen gas:
M(s) + 2HCl(aq) → MCl₂(aq) + H₂(g)
For instance, magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Applications and Uses of Alkaline Earth Metals: A Diverse Range
The alkaline earth metals and their compounds find diverse applications across various industries. Let's examine some prominent uses:
Magnesium (Mg): A Lightweight Champion
Magnesium is known for its lightweight yet strong nature, making it indispensable in various applications:
- Automotive Industry: Used in lightweight alloys for car parts, reducing fuel consumption.
- Aerospace Industry: Utilized in aircraft components for weight reduction.
- Biomedical Applications: Magnesium alloys are used in biodegradable implants and stents.
- Photography: Used in flashbulbs due to its bright combustion.
Calcium (Ca): Essential for Life and Industry
Calcium is an essential nutrient for living organisms, playing a vital role in bone structure and various biological processes. Industrially:
- Building Materials: Calcium carbonate (CaCO₃), in the form of limestone and marble, is a major component of cement and concrete.
- Plaster and Gypsum: Calcium sulfate (CaSO₄) is used in plaster and gypsum products.
- Agriculture: Calcium compounds are used as fertilizers to improve soil fertility.
Strontium (Sr) and Barium (Ba): Specialized Applications
Strontium and barium find specialized applications:
-
Strontium: Used in fireworks to produce red flames. Also used in certain alloys and as a component in some types of glass.
-
Barium: Barium sulfate (BaSO₄) is used as a contrast agent in medical imaging (X-rays). Barium compounds are also used in certain types of ceramics and glass.
Beryllium (Be): A Unique and Challenging Element
Beryllium is a unique element with some special properties and challenges:
-
High Strength-to-Weight Ratio: Its remarkable strength and lightweight nature make it valuable in aerospace and military applications. It's used in high-speed aircraft and missiles.
-
Toxicity: Beryllium is toxic, requiring careful handling and safety precautions in industrial settings.
Radium (Ra): A Radioactive Element
Radium is a radioactive element, with limited applications due to its radioactivity:
- Historical Use: It was once used in luminous paints, but this application has been discontinued due to safety concerns.
Environmental Considerations and Toxicity
While the alkaline earth metals play crucial roles in various applications, it's essential to acknowledge their environmental impacts and potential toxicity. Beryllium, for example, is highly toxic, posing health risks through inhalation. Proper handling and disposal are crucial to minimize environmental damage and protect human health. Sustainable practices in mining and industrial processes are essential to ensure responsible utilization of these elements.
Conclusion: The Enduring Importance of Alkaline Earth Metals
The alkaline earth metals are an integral part of the periodic table and our daily lives. Their unique properties, reactivity, and diverse applications showcase their importance across various scientific and technological fields. From the construction industry to biomedical applications, these elements have significantly impacted modern society. Understanding their characteristics, reactions, and uses is crucial for continued innovation and sustainable development. Ongoing research continues to unravel new insights into these fascinating elements, highlighting their enduring importance in science and technology.
Latest Posts
Latest Posts
-
Equilibrium Is Reached When What Occurs
Mar 30, 2025
-
What Is The Relationship Between Energy And Frequency
Mar 30, 2025
-
Does Liquid Have A Definite Shape And Volume
Mar 30, 2025
-
What Happens To Electrons In A Covalent Bond
Mar 30, 2025
-
Which Part Of An Amino Acid Is Always Acidic
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Group 2a Elements Are Also Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
