How Many Electrons Are In Cl-
Muz Play
Mar 28, 2025 · 5 min read
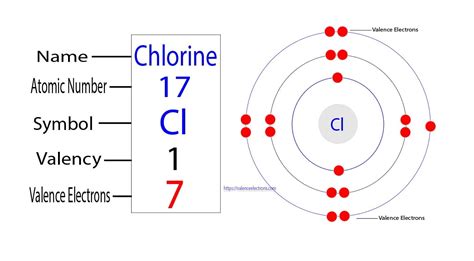
Table of Contents
How Many Electrons Are in Cl-? Understanding Chloride Ions
The seemingly simple question, "How many electrons are in Cl-?" opens a door to a deeper understanding of atomic structure, chemical bonding, and the behavior of ions. This article will delve into the answer, exploring the concepts needed to fully grasp the electron count in a chloride ion (Cl⁻) and its implications. We'll also explore related concepts such as atomic number, isotopes, and ionic bonding.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we can determine the number of electrons in a chloride ion, we need to understand the fundamental building blocks of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom, maintaining electrical neutrality.
Chlorine (Cl): Atomic Number and Electron Configuration
Chlorine (Cl) is a chemical element with an atomic number of 17. This means a neutral chlorine atom contains 17 protons in its nucleus. Consequently, a neutral chlorine atom also has 17 electrons orbiting the nucleus. These electrons are distributed across different energy levels or shells according to the rules of electron configuration:
- First shell (n=1): Holds a maximum of 2 electrons.
- Second shell (n=2): Holds a maximum of 8 electrons.
- Third shell (n=3): Holds a maximum of 18 electrons (though chlorine only populates a portion of this shell).
Therefore, the electron configuration of a neutral chlorine atom is 1s²2s²2p⁶3s²3p⁵. This notation indicates the number of electrons in each subshell. The superscripts represent the number of electrons in each subshell.
The Chloride Ion (Cl⁻): Gaining an Electron
Chlorine, being in Group 17 (also known as the halogens), is highly reactive. It readily gains one electron to achieve a stable electron configuration. This stable configuration, known as an octet, involves having eight electrons in its outermost shell (valence shell). By gaining one electron, chlorine achieves a full octet in its third shell.
This process of gaining an electron results in the formation of a chloride ion (Cl⁻). The extra electron gives the ion a net negative charge, hence the negative sign.
How Many Electrons in Cl-? The Answer
Therefore, a chloride ion (Cl⁻) has 18 electrons. This is one more electron than the neutral chlorine atom (17 electrons + 1 gained electron = 18 electrons). Its electron configuration becomes 1s²2s²2p⁶3s²3p⁶, a stable octet configuration.
Isotopes and their Effect on Electron Count
While the number of protons defines an element, the number of neutrons can vary. These variations create isotopes of the same element. For instance, chlorine has two naturally occurring isotopes: ³⁵Cl and ³⁷Cl. The superscript represents the mass number (protons + neutrons).
Despite having different numbers of neutrons, the number of electrons in both isotopes of chlorine remains the same in their neutral states (17 electrons). When they form chloride ions, both gain one electron to become Cl⁻, each having 18 electrons. The isotopic variation affects the mass but not the electron configuration or the ionic charge.
Ionic Bonding and the Chloride Ion
The formation of the chloride ion is a crucial example of ionic bonding. Ionic bonding occurs when one atom (in this case, chlorine) gains electrons, becoming an anion (negatively charged ion), and another atom loses electrons, becoming a cation (positively charged ion). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Many common salts, like sodium chloride (NaCl), are formed through ionic bonding. In NaCl, sodium (Na) loses one electron to become Na⁺, and chlorine gains that electron to become Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ creates the ionic crystal structure of table salt.
Importance of the Chloride Ion in Biology and Chemistry
Chloride ions play critical roles in various biological and chemical processes. In biology:
- Maintaining fluid balance: Cl⁻ is a major anion in extracellular fluids, contributing to osmotic pressure and fluid balance.
- Nerve impulse transmission: Cl⁻ channels regulate the electrical potential across cell membranes, crucial for nerve impulse transmission.
- Digestion: Hydrochloric acid (HCl), a strong acid containing chloride ions, is essential for digestion in the stomach.
In chemistry:
- Formation of various compounds: Cl⁻ participates in the formation of numerous inorganic and organic compounds, many with industrial applications.
- Electrolyte solutions: Chloride ions are important components in electrolyte solutions, utilized in various applications including batteries.
Conclusion: The Significance of Electron Count in Chemical Behavior
Understanding the number of electrons in an ion, like Cl⁻, is fundamental to comprehending its chemical behavior. The gain of a single electron dramatically alters chlorine's properties, transforming a highly reactive gas into a stable anion that plays crucial roles in various biological and chemical processes. This seemingly simple calculation highlights the fundamental principles of atomic structure and chemical bonding, emphasizing the critical role of electron configuration in determining the properties and reactivity of elements and their ions. The 18 electrons in Cl⁻ are not merely a number; they represent the stability achieved through ionic bonding and the consequent chemical and biological significance of this important ion.
Latest Posts
Latest Posts
-
What Is End Point In Titration
Mar 31, 2025
-
How Do You Balance A Nuclear Equation
Mar 31, 2025
-
Buffer Solution Of Acetic Acid And Sodium Acetate
Mar 31, 2025
-
Choose The Correct Names Of The Atoms Or Molecules
Mar 31, 2025
-
Integrals Of Even And Odd Functions
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Cl- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
