How To Calculate Molar Solubility From Ksp
Muz Play
Mar 28, 2025 · 5 min read
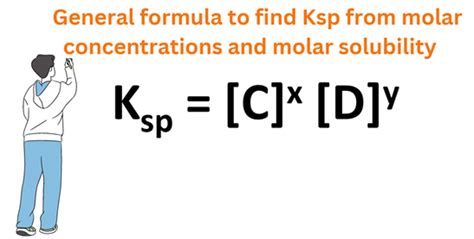
Table of Contents
How to Calculate Molar Solubility from Ksp: A Comprehensive Guide
The solubility product constant, Ksp, is a crucial concept in chemistry that quantifies the solubility of sparingly soluble ionic compounds. Understanding how to calculate molar solubility from Ksp is essential for various applications, from predicting precipitation reactions to analyzing environmental samples. This comprehensive guide will walk you through the process, covering various scenarios and providing examples to solidify your understanding.
Understanding Ksp and Molar Solubility
Before diving into the calculations, let's clarify the fundamental terms:
Ksp (Solubility Product Constant): This equilibrium constant represents the product of the concentrations of the ions raised to their stoichiometric coefficients in a saturated solution of a sparingly soluble salt. A higher Ksp value indicates greater solubility, while a lower Ksp value signifies lower solubility. It's important to remember that Ksp is temperature-dependent; changes in temperature will affect the value of Ksp.
Molar Solubility: This refers to the number of moles of solute that can dissolve in one liter of a saturated solution at a given temperature. It represents the maximum amount of a substance that can dissolve before the solution becomes saturated and precipitation occurs. Molar solubility is usually expressed in moles per liter (mol/L) or M.
Calculating Molar Solubility from Ksp: Simple Cases
The simplest calculations involve ionic compounds that dissociate completely into their constituent ions. Let's illustrate this with examples:
Example 1: Silver Chloride (AgCl)
Silver chloride is a sparingly soluble salt that dissociates as follows:
AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
The Ksp expression for AgCl is:
Ksp = [Ag⁺][Cl⁻]
If 's' represents the molar solubility of AgCl, then at equilibrium:
[Ag⁺] = s [Cl⁻] = s
Therefore, the Ksp expression becomes:
Ksp = s²
To find the molar solubility (s), simply take the square root of Ksp:
s = √Ksp
**Example 2: Lead(II) Iodide (PbI₂) **
Lead(II) iodide dissociates as follows:
PbI₂(s) ⇌ Pb²⁺(aq) + 2I⁻(aq)
The Ksp expression is:
Ksp = [Pb²⁺][I⁻]²
If 's' is the molar solubility of PbI₂, then at equilibrium:
[Pb²⁺] = s [I⁻] = 2s
Substituting into the Ksp expression:
Ksp = s(2s)² = 4s³
Solving for 's':
s = ³√(Ksp/4)
Calculating Molar Solubility from Ksp: More Complex Scenarios
The examples above represent straightforward cases. Let's explore scenarios that introduce more complexity:
1. Common Ion Effect:
The presence of a common ion in the solution significantly reduces the solubility of a sparingly soluble salt. This is known as the common ion effect. Let's consider the solubility of AgCl in a solution containing 0.1 M NaCl.
The dissociation of AgCl remains the same:
AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
Ksp = [Ag⁺][Cl⁻]
However, the chloride ion concentration is no longer solely determined by the solubility of AgCl. We have:
[Cl⁻] = 0.1 M + s
Assuming that 's' is much smaller than 0.1 M (a valid approximation for sparingly soluble salts), we can simplify this to:
[Cl⁻] ≈ 0.1 M
Therefore:
Ksp = [Ag⁺][Cl⁻] ≈ s(0.1)
Solving for s:
s = Ksp / 0.1
2. pH Effects:
The solubility of some sparingly soluble salts is affected by the pH of the solution. This is particularly true for salts of weak acids or bases. For instance, consider the solubility of calcium hydroxide, Ca(OH)₂.
Ca(OH)₂(s) ⇌ Ca²⁺(aq) + 2OH⁻(aq)
Ksp = [Ca²⁺][OH⁻]²
In a basic solution, the concentration of hydroxide ions ([OH⁻]) is increased, shifting the equilibrium to the left and decreasing the solubility of Ca(OH)₂. Conversely, in an acidic solution, the increased concentration of H⁺ ions will react with OH⁻ ions, decreasing [OH⁻] and increasing the solubility of Ca(OH)₂. To solve such problems, you'll need to incorporate the hydroxide or hydronium ion concentration from the pH into the Ksp expression.
3. Complex Ion Formation:
The solubility of some metal salts can be significantly enhanced by the formation of complex ions. For example, the solubility of AgCl increases dramatically in the presence of ammonia due to the formation of the diamminesilver(I) complex ion, [Ag(NH₃)₂]⁺. The complete equilibrium is a combination of the solubility equilibrium and the complex ion formation equilibrium.
AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
Ag⁺(aq) + 2NH₃(aq) ⇌ [Ag(NH₃)₂]⁺(aq)
This requires a more complex approach, incorporating the formation constant (Kf) of the complex ion into the calculation of the overall solubility.
Advanced Techniques and Considerations
1. Iterative Methods:
In certain situations, particularly when the assumption that 's' is negligible compared to the common ion concentration is invalid, iterative methods might be necessary to solve for 's' accurately. These methods involve repeatedly refining the estimate of 's' until a consistent value is obtained.
2. Activity Coefficients:
In highly concentrated solutions, the ionic strength significantly affects the activity of ions. To obtain accurate results, activity coefficients must be included in the Ksp calculations, replacing concentrations with activities. This adds complexity to the calculations but is crucial for precise results in high-ionic-strength solutions.
3. Limitations of Ksp:
It's vital to understand that Ksp values provide an idealized representation of solubility. Several factors, such as the presence of other ions, temperature fluctuations, and the formation of ion pairs or complexes, can affect the actual solubility. Therefore, Ksp calculations should be viewed as estimations that provide valuable insights into the solubility behavior of ionic compounds.
Practical Applications
The ability to calculate molar solubility from Ksp has several practical applications in various fields, including:
- Qualitative Analysis: Predicting whether a precipitate will form when two solutions are mixed.
- Environmental Chemistry: Assessing the concentration of heavy metal ions in water systems.
- Pharmaceutical Sciences: Determining the solubility of drugs and their bioavailability.
- Geochemistry: Understanding the solubility of minerals and their distribution in geological formations.
- Material Science: Designing and synthesizing new materials with specific solubility characteristics.
Conclusion
Calculating molar solubility from Ksp is a fundamental skill in chemistry with far-reaching applications. While simple cases involve straightforward calculations, more complex scenarios require a deeper understanding of the common ion effect, pH effects, complex ion formation, and other factors that can influence solubility. Mastering these concepts empowers you to interpret and predict the behavior of sparingly soluble ionic compounds in various environments. This guide has provided a solid foundation, but further exploration and practice with diverse examples will strengthen your understanding and problem-solving abilities. Remember always to consider the limitations of Ksp and choose the appropriate calculation method depending on the complexity of the situation.
Latest Posts
Latest Posts
-
What Is End Point In Titration
Mar 31, 2025
-
How Do You Balance A Nuclear Equation
Mar 31, 2025
-
Buffer Solution Of Acetic Acid And Sodium Acetate
Mar 31, 2025
-
Choose The Correct Names Of The Atoms Or Molecules
Mar 31, 2025
-
Integrals Of Even And Odd Functions
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Molar Solubility From Ksp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
