Is Carbon Dioxide A Pure Substance Or A Mixture
Muz Play
Mar 27, 2025 · 5 min read
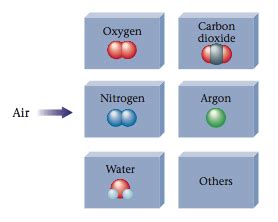
Table of Contents
Is Carbon Dioxide a Pure Substance or a Mixture?
The question of whether carbon dioxide (CO2) is a pure substance or a mixture often arises in chemistry discussions. Understanding this requires a clear grasp of the definitions of pure substances and mixtures. This article will delve deep into the chemical nature of carbon dioxide, exploring its composition, properties, and how these characteristics classify it within the context of chemistry's fundamental classifications. We'll also address some common misconceptions and delve into related concepts to provide a comprehensive understanding.
Defining Pure Substances and Mixtures
Before we classify carbon dioxide, let's establish the definitions of pure substances and mixtures. This fundamental understanding is crucial for the accurate classification of any material.
Pure Substances
A pure substance is a form of matter that has a constant chemical composition and distinct chemical properties. This means that it's made up of only one type of atom or molecule. Pure substances cannot be separated into simpler components through physical methods like filtration or distillation. They have a fixed melting point and boiling point. Examples include:
- Elements: These are substances made up of only one type of atom, such as oxygen (O), iron (Fe), or gold (Au).
- Compounds: These are substances composed of two or more different atoms chemically bonded in a fixed ratio. Examples include water (H₂O), salt (NaCl), and glucose (C₆H₁₂O₆).
Mixtures
A mixture, on the other hand, is a combination of two or more substances that are not chemically bonded. Mixtures can be separated into their individual components using physical methods. The composition of a mixture is not fixed and can vary. There are two main types of mixtures:
- Homogeneous mixtures: These have a uniform composition throughout. The individual components are not visibly distinguishable. Examples include saltwater, air, and sugar dissolved in water.
- Heterogeneous mixtures: These have a non-uniform composition. The different components are visibly distinguishable. Examples include sand and water, oil and water, and a salad.
The Chemical Composition of Carbon Dioxide
Carbon dioxide (CO2) is a chemical compound, not a mixture. It's formed from the chemical bonding of one carbon atom (C) and two oxygen atoms (O). This bonding is a covalent bond, where the atoms share electrons to achieve a stable electron configuration. This specific 1:2 ratio of carbon to oxygen atoms is always constant in pure carbon dioxide. You cannot find a sample of CO2 with a different ratio without it being a different substance.
The Covalent Bond in CO2
The covalent bond in CO2 is a crucial aspect determining its classification as a pure substance. This strong chemical bond holds the carbon and oxygen atoms together, forming a distinct molecule. This molecular structure dictates the physical and chemical properties of CO2.
Key Characteristics of CO2's Covalent Bond:
- Strong Bond: The covalent bonds within a CO2 molecule are relatively strong, requiring significant energy to break them.
- Linear Structure: The molecule adopts a linear structure with the carbon atom in the center and the two oxygen atoms on either side.
- Polarity: Although the individual C=O bonds are polar (due to the difference in electronegativity between carbon and oxygen), the overall molecule is nonpolar due to its linear symmetry. This influences CO2's interactions with other molecules.
Why CO2 is Not a Mixture
Several factors definitively classify CO2 as a pure substance, not a mixture:
- Fixed Composition: CO2 always has a fixed ratio of one carbon atom to two oxygen atoms. This consistent stoichiometry is a hallmark of pure compounds.
- Distinct Properties: CO2 possesses unique physical and chemical properties (e.g., melting point, boiling point, density, reactivity) that distinguish it from its constituent elements (carbon and oxygen). These properties are consistent for pure CO2.
- Inability to Separate Components Physically: You cannot physically separate carbon and oxygen from CO2 by simple methods like filtration or distillation. Chemical processes are required to break the strong covalent bonds.
- Uniform Composition: A sample of pure CO2 will have a uniform composition throughout, unlike mixtures, which can have varying compositions.
Common Misconceptions
Some misunderstandings may arise concerning the classification of CO2. Let's clarify some of these:
- Air Contains CO2: Air is a mixture containing various gases, including carbon dioxide. However, this does not mean CO2 itself is a mixture. CO2 remains a pure substance even within the context of a mixture like air.
- CO2 in Different States: CO2 can exist in various states (solid, liquid, gas), but its chemical composition remains unchanged regardless of its physical state. The state changes are purely physical transformations and do not alter the substance's chemical nature.
- Impurities in CO2: While commercially produced CO2 may contain trace impurities, a truly pure sample of CO2 consists solely of CO2 molecules. The presence of impurities does not change its fundamental classification.
Further Exploration: Related Concepts
Understanding the classification of CO2 helps illuminate related concepts in chemistry:
- Law of Definite Proportions: This law states that a pure compound always contains the same elements in the same proportion by mass. CO2 perfectly exemplifies this law with its consistent 1:2 ratio of carbon to oxygen.
- Chemical Formulas: The chemical formula for CO2 concisely represents the composition of the molecule, showing the types and numbers of atoms present.
- Molecular Weight: The molecular weight of CO2 (approximately 44 g/mol) is a direct result of its chemical composition and is a characteristic property of the pure substance.
Conclusion
In conclusion, carbon dioxide (CO2) is definitively a pure substance, specifically a compound. Its fixed chemical composition, distinct properties, and the strong covalent bonds holding its atoms together all point to this classification. Understanding this fundamental concept helps solidify the understanding of chemical classification and the properties of matter. While CO2 may exist within mixtures (such as air), the substance itself remains a pure compound with a consistent molecular structure and properties. The presence of impurities in commercially available CO2 does not alter its classification as a pure substance, provided those impurities are present in trace amounts and do not change the overall molecular composition significantly.
Latest Posts
Latest Posts
-
Why Are More Substituted Alkenes More Stable
Mar 30, 2025
-
How Does Reactivity Of Metals Increase
Mar 30, 2025
-
Equilibrium Is Reached When What Occurs
Mar 30, 2025
-
What Is The Relationship Between Energy And Frequency
Mar 30, 2025
-
Does Liquid Have A Definite Shape And Volume
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Carbon Dioxide A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
