Movement Of Particles From High To Low Concentration
Muz Play
Mar 29, 2025 · 7 min read
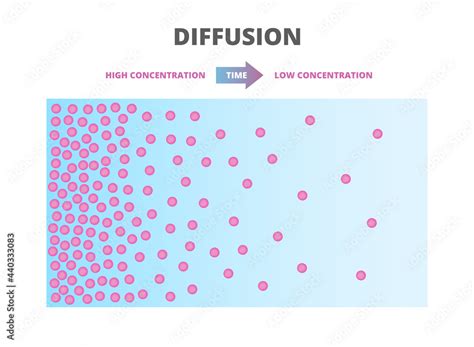
Table of Contents
Movement of Particles from High to Low Concentration: A Deep Dive into Diffusion and Osmosis
The fundamental principle governing the movement of particles from regions of high concentration to regions of low concentration is a cornerstone of numerous biological and physical processes. This movement, driven by the inherent tendency of systems to achieve equilibrium, manifests itself in various forms, most notably diffusion and osmosis. Understanding these processes is critical to grasping the complexities of everything from cellular respiration to the movement of pollutants in the environment. This comprehensive article delves into the mechanics of this movement, exploring the factors that influence it and providing real-world examples.
What is Diffusion?
Diffusion is the net passive movement of particles (atoms, ions, or molecules) from a region of higher concentration to a region of lower concentration. This movement continues until the particles are uniformly distributed throughout the available space. This process is driven by the random thermal motion of particles; they are constantly colliding and moving in random directions. In a region of high concentration, the particles are more crowded, resulting in a higher probability of particles moving towards the less crowded area of lower concentration.
Factors Affecting Diffusion Rate
Several factors influence the rate at which diffusion occurs:
-
Concentration Gradient: The steeper the concentration gradient (the greater the difference in concentration between two regions), the faster the rate of diffusion. A larger difference means more particles are available to move from the high-concentration area.
-
Temperature: Higher temperatures increase the kinetic energy of particles, leading to faster movement and thus a higher diffusion rate. The particles collide more frequently and with greater force.
-
Particle Size and Mass: Smaller and lighter particles diffuse faster than larger and heavier ones. This is because they are less resistant to changes in motion.
-
Distance: The rate of diffusion is inversely proportional to the distance over which diffusion occurs. Diffusion is more efficient over shorter distances.
-
Medium: The medium through which diffusion occurs plays a significant role. Diffusion is faster in gases than in liquids, and slower in solids. The density and viscosity of the medium affect the ease with which particles can move.
-
Surface Area: A larger surface area available for diffusion allows more particles to move simultaneously, leading to a faster rate. Think of the difference between a small cube of sugar dissolving versus a large cube.
Examples of Diffusion
Diffusion is a ubiquitous process found throughout nature and technology:
-
Oxygen and Carbon Dioxide Exchange in the Lungs: Oxygen diffuses from the alveoli (air sacs) in the lungs into the bloodstream, while carbon dioxide diffuses from the blood into the alveoli to be exhaled. The steep concentration gradients ensure efficient gas exchange.
-
Nutrient Absorption in the Intestines: Nutrients from digested food diffuse from the intestines into the bloodstream, providing the body with the necessary building blocks and energy.
-
Fragrance Spreading in a Room: The pleasant smell of a perfume or a freshly baked cake spreads throughout a room through diffusion of volatile aromatic molecules.
-
Dye Dispersion in Water: If you drop a drop of food coloring into a glass of water, you'll observe the dye gradually dispersing until the water is uniformly colored. This is a classic example of diffusion in action.
-
Fertilizer Uptake by Plants: Plants absorb nutrients from the soil through diffusion. Fertilizers increase the concentration of nutrients in the soil, facilitating their uptake by plant roots.
What is Osmosis?
Osmosis is a special case of diffusion that involves the movement of water molecules across a selectively permeable membrane. A selectively permeable membrane is a barrier that allows some substances to pass through but restricts the passage of others. In osmosis, water moves from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). The movement continues until the water potential is equal on both sides of the membrane.
Water Potential and Osmotic Pressure
-
Water Potential: This refers to the tendency of water to move from one area to another. Pure water has the highest water potential. Adding solutes to water lowers its water potential.
-
Osmotic Pressure: This is the pressure required to prevent the net movement of water across a selectively permeable membrane. The higher the solute concentration, the higher the osmotic pressure.
Types of Osmotic Solutions
When comparing the solute concentration of a solution to that of a cell, we use three terms to describe the solution:
-
Hypotonic Solution: A solution with a lower solute concentration than the cell. Water will move into the cell, causing it to swell and potentially burst (lyse).
-
Hypertonic Solution: A solution with a higher solute concentration than the cell. Water will move out of the cell, causing it to shrink (crenate).
-
Isotonic Solution: A solution with the same solute concentration as the cell. There is no net movement of water.
Examples of Osmosis
Osmosis plays a crucial role in many biological processes:
-
Water Uptake by Plant Roots: Water moves from the soil (hypotonic) into the roots of plants (hypertonic) through osmosis. This process is essential for plant growth and survival.
-
Water Reabsorption in the Kidneys: The kidneys regulate water balance in the body through osmosis. Water is reabsorbed from the filtrate back into the bloodstream.
-
Maintaining Cell Turgor Pressure: Osmosis helps maintain the turgor pressure (internal pressure) in plant cells, keeping them firm and upright.
-
Water Movement Across Cell Membranes: Osmosis is vital for maintaining the proper water balance within cells. The cells must constantly regulate their water content to avoid shrinking or bursting.
-
Dehydration: If you don't drink enough water, the concentration of solutes in your blood increases. This causes water to move out of your cells, leading to dehydration.
Diffusion vs. Osmosis: Key Differences
While both diffusion and osmosis involve the movement of particles from high to low concentration, they differ in several key aspects:
| Feature | Diffusion | Osmosis |
|---|---|---|
| Substance | Any substance | Primarily water |
| Membrane | May or may not involve a membrane | Always involves a selectively permeable membrane |
| Driving Force | Concentration gradient | Water potential gradient |
| Specificity | Non-specific | Specific to water |
Facilitated Diffusion
Facilitated diffusion is a type of passive transport where molecules move across a membrane with the assistance of membrane proteins. These proteins act as channels or carriers, facilitating the movement of specific molecules down their concentration gradient. This process is still passive because it doesn't require energy input. It speeds up the rate of diffusion for molecules that would otherwise cross the membrane very slowly or not at all.
Examples of Facilitated Diffusion
-
Glucose Transport: Glucose, an essential sugar for cellular respiration, enters cells through facilitated diffusion using glucose transporter proteins.
-
Ion Transport: Many ions, such as sodium, potassium, and calcium, are transported across cell membranes via facilitated diffusion using ion channels.
Active Transport
Unlike diffusion and osmosis, which are passive processes, active transport requires energy input to move molecules against their concentration gradient—from an area of low concentration to an area of high concentration. This energy is usually supplied by ATP (adenosine triphosphate). Active transport is essential for maintaining concentration gradients that are necessary for many cellular processes.
Examples of Active Transport
-
Sodium-Potassium Pump: This pump maintains the concentration gradient of sodium and potassium ions across the cell membrane, crucial for nerve impulse transmission and muscle contraction.
-
Endocytosis and Exocytosis: These processes involve the bulk transport of materials into and out of cells, respectively. They require energy and are considered forms of active transport.
Conclusion
The movement of particles from high to low concentration, whether through diffusion, osmosis, or facilitated diffusion, is a fundamental principle driving countless biological and physical processes. Understanding these mechanisms is crucial for comprehending the workings of cells, organisms, and the environment as a whole. The factors influencing these processes, such as concentration gradients, temperature, and membrane permeability, provide valuable insights into the dynamic equilibrium that governs the distribution of matter. Furthermore, contrasting passive transport with the energy-requiring active transport highlights the diversity and complexity of transport mechanisms crucial for life. This knowledge forms the basis for many advancements in fields like medicine, agriculture, and environmental science. Further exploration of these topics will undoubtedly unveil deeper understanding and lead to innovative applications in the future.
Latest Posts
Latest Posts
-
Potential And Kinetic Energy In A Pendulum
Mar 31, 2025
-
Kinetic Energy Of An Ideal Gas
Mar 31, 2025
-
How To Find Ms Quantum Number
Mar 31, 2025
-
Packing Efficiency Of Face Centered Cubic
Mar 31, 2025
-
Diisopropyl Ether Reacts With Concentrated Aqueous Hi
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Movement Of Particles From High To Low Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
