What Are The Units Of Entropy
Muz Play
Mar 28, 2025 · 6 min read
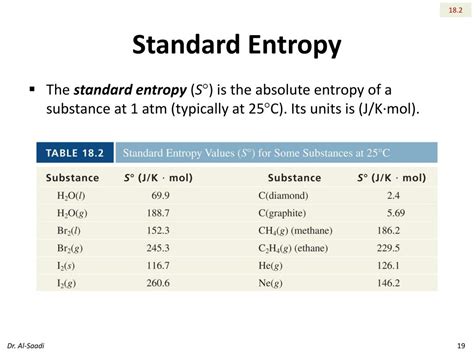
Table of Contents
What Are the Units of Entropy? A Deep Dive into Thermodynamic and Information Theory
Entropy, a cornerstone concept in both thermodynamics and information theory, measures disorder or randomness within a system. While seemingly disparate fields, thermodynamics and information theory share a surprisingly elegant connection through the unifying concept of entropy. However, understanding the units of entropy requires appreciating its different contexts and interpretations. This article provides a comprehensive overview, exploring the units used to quantify entropy in each field and the subtle yet significant distinctions between them.
Entropy in Thermodynamics: A Measure of Disorder
In thermodynamics, entropy (often denoted as S) quantifies the degree of randomness or disorder within a physical system. A system with high entropy is highly disordered, while a system with low entropy is highly ordered. The second law of thermodynamics states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. This signifies the universe's natural tendency towards greater disorder.
The Joule per Kelvin (J/K)
The standard unit of entropy in thermodynamics is the joule per kelvin (J/K). This unit arises directly from the fundamental thermodynamic definition of entropy:
dS = δQ<sub>rev</sub>/T
Where:
- dS represents an infinitesimal change in entropy.
- δQ<sub>rev</sub> represents an infinitesimal amount of heat added reversibly to the system. The reversibility condition is crucial, as irreversible processes increase entropy beyond what's accounted for by this equation.
- T represents the absolute temperature of the system in Kelvin.
This equation highlights the core relationship: the change in entropy is proportional to the heat added and inversely proportional to the temperature. Adding a small amount of heat to a hot system (high T) causes a smaller increase in entropy than adding the same amount of heat to a cold system (low T). Since heat (δQ<sub>rev</sub>) is measured in joules (J) and temperature (T) in Kelvin (K), the resulting unit for entropy is J/K.
Understanding the Implications of J/K
The J/K unit reflects the fundamental connection between heat transfer and disorder. When heat is added to a system, the increased energy allows for more microscopic configurations (more possible arrangements of particles), thus increasing disorder and entropy. The temperature dependence underscores that the same amount of heat has a greater impact on disorder at lower temperatures. Consider a perfectly ordered crystal at absolute zero (0 K): adding any heat, however small, results in a significant increase in entropy because the system transitions from a state of perfect order to one of increased disorder.
Beyond J/K: Specific Entropy and Other Considerations
While J/K is the fundamental unit, you often encounter entropy expressed differently depending on the specific application:
- Specific entropy (s): This represents the entropy per unit mass (J/kg·K). It's useful when dealing with extensive systems where the total entropy is proportional to the mass.
- Molar entropy (S<sub>m</sub>): This is the entropy per mole of substance (J/mol·K). It's valuable when comparing the entropic properties of different substances on a molar basis.
Entropy in Information Theory: A Measure of Uncertainty
In information theory, entropy (often denoted as H) quantifies the uncertainty or randomness associated with a random variable. It measures the average amount of information needed to describe the outcome of a random variable. A higher entropy indicates greater uncertainty, while a lower entropy indicates less uncertainty. This is a different, yet mathematically analogous, concept to thermodynamic entropy.
The Bit (or Nat, or Dit)
The primary unit of information entropy in information theory is the bit. One bit represents the amount of information gained when the uncertainty about a binary event (e.g., a coin flip) is resolved. However, other units are also used, depending on the base of the logarithm used in the defining equation:
- Bit: Based on base-2 logarithms (log₂). This is the most common unit in computer science and digital communication.
- Nat: Based on natural logarithms (ln). This is frequently used in theoretical contexts.
- Dit (or hartley): Based on base-10 logarithms (log₁₀). Less frequently encountered than bits or nats.
Shannon's Entropy Formula and its Units
Claude Shannon's groundbreaking work formalized information entropy with the following formula:
H(X) = - Σ P(xᵢ) log₂ P(xᵢ)
Where:
- H(X) represents the Shannon entropy of the random variable X.
- P(xᵢ) represents the probability of the i-th outcome of the random variable X.
- The summation is taken over all possible outcomes of X.
This formula uses base-2 logarithms, hence the resulting unit is bits. Each term in the summation represents the contribution of a particular outcome to the overall uncertainty. Outcomes with higher probability contribute less to the entropy than those with lower probability, reflecting the intuition that less surprising events provide less information.
The Connection Between Thermodynamic and Information Entropy
While the units differ (J/K vs. bits), the mathematical structures of thermodynamic and information entropy are strikingly similar. Both quantify uncertainty or randomness, albeit in different contexts. The Boltzmann constant (k<sub>B</sub>) provides a bridge between the two, allowing a conversion factor between the units:
k<sub>B</sub> ≈ 1.38 × 10<sup>-23</sup> J/K
This constant's significance lies in its ability to relate the microscopic level (number of microstates in a thermodynamic system) to the macroscopic level (entropy as measured in J/K). This deep connection between these fields underscores the power and universality of the entropy concept.
Beyond Bits: Other Units and Applications
While bits are the dominant unit, other units related to information entropy emerge in specific applications:
- Bits per symbol: This is used when dealing with discrete sources emitting symbols. It represents the average information content per symbol transmitted.
- Bits per second (bps): This quantifies the rate of information transmission. It's crucial in network communication and data storage.
Practical Applications and Examples of Entropy Units
Understanding the units of entropy is crucial for numerous applications across various disciplines:
- Thermodynamics: Calculating the efficiency of heat engines, predicting the spontaneity of chemical reactions, analyzing phase transitions. The J/K unit is fundamental here.
- Statistical mechanics: Linking macroscopic properties to microscopic configurations. The Boltzmann constant plays a vital role in converting between J/K and the number of microstates.
- Information theory: Designing efficient data compression algorithms (e.g., Huffman coding), evaluating the capacity of communication channels, analyzing cryptographic security. Bits (or nats, dits) are central here.
- Machine learning: Measuring the uncertainty in probabilistic models, understanding model complexity. Bits often serve as the unit of choice for quantifying uncertainty.
- Ecology: Assessing biodiversity and ecosystem stability. The connection between information entropy and biodiversity allows for quantifying ecosystem health.
Conclusion: A Unified View of Entropy
Entropy, whether in thermodynamics or information theory, fundamentally measures disorder and uncertainty. While the units (J/K and bits) might seem different at first glance, they stem from mathematically analogous frameworks that provide powerful tools across diverse fields. Understanding these units and their context is essential for accurately interpreting and applying entropy calculations in various scientific, engineering, and technological endeavors. The seemingly simple units encapsulate deep concepts with wide-ranging implications across many disciplines, highlighting the universality of the concept of entropy.
Latest Posts
Latest Posts
-
Buffer Solution Of Acetic Acid And Sodium Acetate
Mar 31, 2025
-
Choose The Correct Names Of The Atoms Or Molecules
Mar 31, 2025
-
Integrals Of Even And Odd Functions
Mar 31, 2025
-
What Makes Something A Strong Acid
Mar 31, 2025
-
According To The Rules Of Osmosis A System Will
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are The Units Of Entropy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
